| |
12)
Endocrinology
A detailed study of fecal estrogen and progesterone
secretion in Grevy's zebra was undertaken by Asa et al. (2001) as well as
Kirkpatrick et al. (1990) Asa et al. also studied the urinary gonadotropin
(eCG) excretion. Progesterone levels were found to be relatively low when
compared with those of numerous other species. Urinary estrone levels became
elevated and were then diagnostic of pregnancy at about 8 months before
the end of pregnancy (Czekala et al., 1990). In horses, it showed a rise
in serum levels as early as the 40th day of gestation. Higher levels were
found in horses than in Hartmann's zebras. Asa et al. (2001) found eCG (equine
chorionic gonadotropin) to be present after 35-40 days, as is the case in
the domestic horse. It completely disappeared at 195 days of their 425 day
gestation. The steroid profiles were similar to those of the horse.
Horses
and, presumably all equidae, have special sites of eCG secretion in their
fetally-derived "endometrial cups". These unique structures
have been studied by numerous investigators, both structurally and functionally,
but they have not been studied in zebras, and their remains are not recognizable
in term placentas. In horses, eCG secretion from these cups begins on
day 32; some time after day 100 the cups are destroyed by an intense maternal
lymphocyte reaction (reviewed in detail by Wooding et al., 2001). While
it is easy to speculate that this hormone may also be the cause of the
fetal gonadal stimulation, its rapid decline after day 100 in maternal
serum argues against this possibility, as the gonads remain large and
apparently stimulated until term. Nevertheless, the unique fetal gonadal
development and the uniqueness of endometrial cups suggest a relationship.
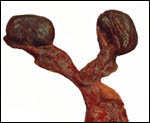
As
is usual for all equidae, the fetal gonads of my Grevy's zebra gestation
were much enlarged and dark brown. They were autolyzed and somewhat diffluent.
They weighed 42 and 39 g.
13) Genetics
The chromosome number of Grevy's zebra is 2n=46;
the Planes' zebras with all their subspecies have 2n=44, and the Mountain
zebras have 2n=32 (Benirschke & Malouf, 1967). Numerous other cytogenetic
studies on the equidae have since appeared, e.g. Gadi & Ryder (1983)
who described the distribution of the nucleolus-organizing regions.
A
wide variety of zebra hybrids have been recorded (Gray, 1972). This includes
hybrids among zebra species, as well as with other equidae. Depending
on their chromosome number, most are sterile offspring (Benirschke et
al., 1964; 1967).
Numerous genetic studies, other than those of the cytogenetic descriptions,
have been done in equidae. For instance, Ryder & Hansen (1979) studied
(G+C)-rich satellites in horses in order to investigate the C-banded regions
of chromosomes. Microsatellite loci of the SINE families were studied
by Gallagher et al. (1999). The mitochondrial DNA evolution of the genus
Equus was investigated by George & Ryder (1986). That study showed
that zebra species diverged most recently from the equid line.
14) Immunology
The most remarkable aspect of an immunologic interaction
between fetus and mare occurs at the implantation site of the chorionic
girdle. Here, the binucleated trophoblast invades the endometrium, partially
destroys it, and thus elicits an immunological maternal response that
ultimately leads to the destruction of the invading trophoblast with cessation
of its eCG secretion. This process appears to be genotypically controlled,
as horse-into-donkey embryo transplants survive, while those of donkey-into-horse
are usually rejected (Enders et al., 1996). This failed pregnancy of the
latter transfers (abortion) appears to be due to the absence of an appropriate
endometrial cup development In part this occurs because of a reduced degree
of trophoblastic invasion, and also because of a more intense early immunological
response. The suggestion that this is genotypically regulated is made.
Perhaps imprinting is another part of this spectrum, as phenotypic sequelae
of imprinting have been described in the phenotypes of hybrids. More complex
transfers are described in this paper, but they are beyond the scope of
this chapter, as zebra studies are still forthcoming.
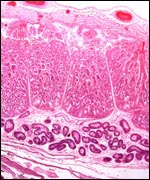
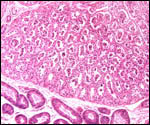
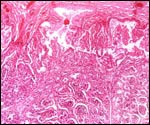
15) Pathological
features
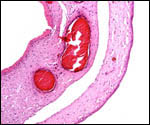
In horses, fetal death has been described as occasionally being due to
excessively twisted, long cords. They have also caused indentations of
the fetal skin. Short cords may rupture and cause demise. One case of
a single umbilical artery was associated with renal anomalies, not unlike
that seen commonly in human gestations (Whitwell, 1975; Benirschke & Kaufmann, 2000).
Griner (1983) listed as principal causes of zebra mortality the following:
Stress and trauma, especially after immobilization; interspecific aggression;
enteritis; volvulus; ascaridiasis; occasional milk aspiration in foals,
and rare intestinal perforation. Neoplastic lesions were not found.
16) Physiologic
data
I know of no relevant zebra studies.
17) Other
resources
Cell lines of these two species, as well as most
other equidae are available from CRES by contacting Dr. Oliver Ryder at:
oryder@ucsd.edu.
18) Other
remarks - What additional Information is needed?
It is highly desirable to obtain early implantational
stages of the zebra placentas with special attention to the development
of endometrial cups. Likewise, studies of eCG secretion the comparison
to other equids are desirable. The secretory output of fetal gonads should
be studied and the stimulus for their development should be investigated.
Acknowledgement
Most of the animal photographs in this chapter come
from the Zoological Society of San Diego. I appreciate also very much
the help of the pathologists at the San Diego Zoo.
References
Amoroso, E.C.: Placentation. In, Marshall's Physiology
of Reproduction, 3rd ed.. A.S. Parkes, ed. V. 2:127-311, 1952, Longmans
Green, London.
Asa,
C.S., Baumann, J.E., Houston, E.W., Fischer, M.T., Read, B., Brownfield,
C.M. and Roser, J.F.: Patterns of excretion of fecal estradiol and progesterone
and urinary chorionic Gonadotropin in Grevy's zebras (Equus grevyi): Ovulatory
cycles and pregnancy. Zoo Biol. 20:185-195, 2001.
Benirschke,
K. and Kaufmann, P.: The Pathology of the Human Placenta. Springer-Verlag,
NY, 2000.
Benirschke,
K., Low, R.J., Brownhill, L.E., Caday, L.B. and de Venecia?Fernandez,
J.: Chromosome studies of a donkey/Grevy zebra hybrid. Chromosoma 15:1?13,
1964. (Benirschke, K.: Corrigendum. Chromosoma 15:300, 1964.)
Benirschke,
K. and Malouf, N.: Chromosome studies of Equidae. In: Equus, Vol. 1 & 2. H. Dathe, ed, pp. 253?284, 1967.
Czekala,
N.M., Kasman, L.H., Allen, J., Oosterhuis, J. and Lasley, B.L.: Urinary
steroid evaluations to monitor ovarian function in exotic ungulates: VI.
Pregnancy detection in exotic equidae. Zoo Biol. 9:43-48, 1990.
Enders,
A.C. and Liu, I.K.M.: Lodgement of the equine blastocyst in the uterus
from fixation through endometrial cup formation. J. Reprod. Fertil. Suppl.
44:427-438, 1991.
Enders,
A.C. and Liu, I.K.M.: Trophoblast-uterine interactions during equine chorionic
girdle cell maturation, migration, and transformation. Amer. J. Anat.
192:366-381, 1991.
Enders,
A.C., Jones, C.J., Lantz, K.C., Schlafke, S. and Liu, I.K.M.: Simultaneous
exocrine and endocrine secretions: trophoblast and glands of the endometrial
cups. J. Reprod. Fertil. Suppl. 56:615-625, 2000.
Enders,
A.C., Meadows, S., Stewart, F. and Allen, W.R.: Failure of endometrial
cup development in the donkey-in-horse model of equine abortion. J. Anat.
188:575-589, 1996.
Gadi,
I.K. and Ryder, O.A.: Distribution of silver-stained nucleolus-organizing
regions in the chromosomes of the Equidae. Genetica 62:109-116, 1983.
Gallagher,
P.C., Lear, T.L., Coogle, L.D. and Bailey, E.: Two SINE families associated
with equine microsatellite loci. Mammalian Genome 10:140-144, 1999.
George,
M. and Ryder, O.A.: Mitochondrial DNA evolution in the genus Equus. Mol.
Biol. Evol. 3:535-546, 1986.
Ginther,
O.J.: Mobility of the early equine conceptus. Theriogenology 19:603-511,
1983.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, 1983.
Keenan,
I.R., Forde, D., McGeady, T., Wande, J. and Roche, J.F.: Endometrial histology
of early pregnant and nonpregnant mares. J. Reprod. Fertil. Suppl. 35:499-504,
1987.
Kirkpatrick,
J.F., Lasley, B.L. and Shideler, S.E.: Urinary steroid evaluations to
monitor ovarian function in exotic ungulates: VII. Urinary progesterone
metabolites in the equidae assessed by immunoassay. Zoo Biol. 9:341-348,
1990.
Mossman,
H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University
of Wisconsin Press, Madison, 1973.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Ryder,
O.A. and Hansen, S.K.: Molecular cytogenetics of the equidae. I. Purification
and cytological localization of a (G+C)-rich satellite DNA from Equus
przewalskii. Chromosoma 72:115-129, 1979.
Whitwell,
K.E.: Morphology and pathology of the equine umbilical cord. J. Reprod.
Fertil. Suppl. 23:599-603, 1975.
Wooding,
F.B.P., Morgan, G., Fowden, A.L. and Allen, W.R.: A structural and immunological
study of chorionic gonadotrophin production by equine trophoblast girdle
and cup cells. Placenta 22:749-767, 2001.
|