| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Aug 20, 2005. |
Phacochoerus africanus sundevallii
Order: Artiodactyla
Family: Suidae
1) General Zoological Data
Suidae had their origin in Eurasia and distributed widely while speciating into significantly different phenotypes and behavioral repertoires. The Suiformes are presumed to be the most primitive of the artiodactyls that formed three major groups: the Suiformes, Tylopoda and Pecora ( Groves , 1981). Warthogs are believed to perhaps be the most recent derivatives. Bosma (1978) reviewed the reasons for placing this species away from the suidae because of their differential dental structures. Nevertheless, the similarity of chromosomes ( v.i. ) argues against this notion. She suggested a recent origin of this species. In his searching discussion of suid development, Thenius (1970) found justification of separating this most recent suid species and he placed the first ancestor into the middle of the Oligocene (20 MYA).
 |
Warthog at San Diego Zoo. |
 |
Warthogs at San Diego Zoo. |
 |
Different species and their facial development (after Thenius, 1970). |
2) General Gestational Data
Gestation of warthogs lasts approximately 140-170 days and litters of 2-6 offspring occur; singletons being rare, quadruplets are born most commonly. Wide variations in gestational length were quoted by Mentis (1972). Newborns weigh between 400 and 500 g. Jones (1993) gave longevity for captive warthogs as 18 years 9 months.
3) Implantation
Initial implantation is accomplished primarily by yolk sac epithelium, which is then the dominant membrane, but it lasts only a short time. There is much uterine “milk” with absorption through this epithelium. By day 24 of the domestic pig pregnancy, the allantois attaches all around the periphery and the yolk sac shrinks (Ramsey, 1982, Mossman, 1987). Additional details of early phases of domestic pig reproduction are available from Geisert (1998) . The uterus is bicornuate and multiple implantations occur in all pigs, first at a mesometrial site. The cord locates mesometrially
4) General Characterization of the Placenta
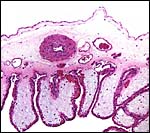
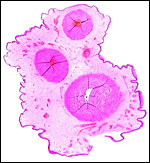
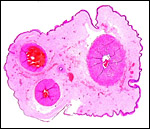
The placentas received come from a birth at San Diego Zoo of 3.1 offspring. They delivered on May/28/2003, the sow having been bred on 12/12/2003 . Thus, the gestation was slightly more than 5 months long. The placentas of two newborns weighed 180 and 130 g. They measured 82 x 14 x 0.5 and 77 x 10 x 0.5 cm. The umbilical cords were 14 x 0.5 and 10 x 0.5 cm in size. As is characteristic of all Suidae (Mossman, 1987) the tips of the placentas had atrophied villi. They were not so necrotic, however, as in the red river hog (see that Chapter). Mossman (1987) described the much more gelatinous nature of pig placentas.
5) Details of fetal/maternal barrier
The placenta of all suidae is a typical, diffuse epitheliochorial placenta without invasion of the endometrium (Mossman1987; Macdonald & Bosma, 1985). The latter authors have compared a number of suid placentas and pregnant uteri, including the warthog. They described the chorion as being rippled and having indentations of trophoblast by capillaries. The single-layered trophoblast interdigitates with endometrial epithelial folds. Mossman (1987) and Macdonald & Bosma (1985) both described the arcades and areolae and the importance of uteroferrin transfer.
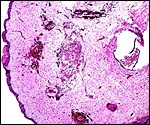
6) Umbilical cord
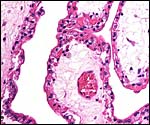
The umbilical cords of these two retrieved placentas measured 14 x 0.5 and 10 x 05. cm. Each contained three large blood vessels and a central patent allantoic duct. In addition, there were numerous small allantoic blood vessels. Small areas of squamous metaplasia were present on their surfaces but they had produced only a minimal amount of keratin. The cords were not spiraled.
7) Uteroplacental circulation
I am not aware on any publications.
8) Extraplacental membranes
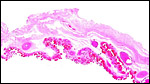
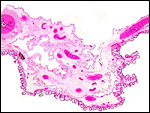
The thin amnion is avascular, as it is usually, and has areas of squamous metaplasia with focal keratin production. The large allantoic sac is vascularized and lined by a thin, single-layered cuboidal to columnar epithelium. No hippomanes were present.
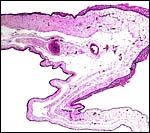 |
Free membranes with amnion on top and vascularized allantois below. Areas of squamous metaplasia are seen on the amnion. |
9) Trophoblast external to barrier
There is no invasion of endometrium by trophoblast.
10) Endometrium
Decidualization does not take place.
11) Various features
None are known to me.
12) Endocrinology
The reproductive cycle is similar to that of Sus scrofa (Eckstein & Zuckerman, 1956). Cycles are about every 21 days, ovulation is spontaneous (not induced), and estrus lasts 2-3 days. These authors also refer to the estrogens and progestins in pregnancy. Progestins are low initially but rise sharply with a peak at 11-15 days and then drop to near zero. Estrogens rise towards term. The duration of pregnancy in the domestic pig is between 112 and 115 days, longer in wild ancestors. Additional information is available from Geisert (1998).
Berger et al. (2002) used fecal steroid metabolites to study the reproductive physiology of warthogs, red river hogs and the babirusa. They were able to define the length of cycles (35-37 days), identify non-cycling sows, and monitor pregnancy.
13) Genetics
The karyology of suids is still incomplete, but an early study by Wallace & Fairall (1967) defined the warthog as having 34 chromosomes. This was confirmed by Bosma (1978) and it is our finding as well. It would appear, as Bosma (1978) inferred, that Robertsonian fusions comprise the major differences among suid karyotype. She reviewed the suggestions of the past to place warthogs into a different line of suids because of the marked difference of dental structure compared with that of other pigs. Nevertheless, the chromosomal similarity argues against this. The domestic pig has 38 chromosomes, but Sus scrofa has two morphotypes (2n=36 or 38, with hybrids having 37), and lower numbers are found in giant forest hog (2n=32), warthog (2n=34). The “bushpig” (red river hog) also has 34 chromosomes (Melander & Hansen-Melander, 1980). The sex chromosomes, as described by these authors, are unusual in their morphology and differ from those of other suids. Specifically, the X-chromosomes of the female specimen that these investigators studied differed in size and appearance. Numerous karyotypes performed of different animals of warthogs and red river hogs in our laboratory have all shown 2n=34 and the X-chromosomes have been large metacentric elements without any structural differences. The red river hog Y-chromosome, however, has a large region of heterochromatin. Bosma et al. (1991) had come to similar conclusions. They studied a “bushpig from the Duisburg Zoo ( Germany ) but did not indicate whether it was a “red river hog” or another subspecies. Upon inquiry, however, it was learned that the animal they studied was in fact a red river hog. They found 34 chromosomes as well. Hybrids of warthogs and red river hogs have not been described. Marczynska & Pigon (1973) described an “African native pig” karyotype (2n=38) but did not exactly specify the species. It is doubtful that they had a bushpig for study. Watanabe et al. (1985) have studied the mtDNA of European and Asiatic pigs. Rothschild & Ruvinsky (198) bring us up-to-date on genetics and immunogenetics of pigs.
 |
Karyotype of female warthog. |
14) Immunology
I am not aware of any studies in warthogs other than the ascertainment of antibodies to some viruses.
15) Pathological features
A variety of helminths have been identified in warthogs from Namibia (Horak et al. 1983). They included cestodes, nine nematode species, six ixodid tick species and argasid tick, lea, louse and larvae of dipteran flies. To this list, Boomker (1990) added infection with Oesophagostomum . Woodall (1989) described dental disease, and Trypanosoma simiae was identified in 11% of warthogs of the Gambia by Claxton et al. (1992). Warthogs are resistant to infection with African swine fever virus (Oura et al. (1998). Stolte et al. (1998) identified two species of Sarcocystis in warthogs. Kenny et al. (2002) reported anemia in a neonatal warthog, identified iron deficiency and suggested iron supplementation in captivity. This type of anemia is common in domestic piglets.
16) Physiologic data
Few physiologic studies have been carried out in this species; nevertheless, numerous veterinary studies on domestic pigs have been done and can be read in the relevant textbooks and in Geisert (1998). Fetal development and pig anatomy are detailed by Patten (1947). Blood chemistries (including cortisol) were reported for warthogs by Keffen et al. (1987).
17) Other resources
Numerous cell strains of warthog fibroblasts are available from CRES at the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu .
18) Other remarks – What additional Information is needed?
Domestic pigs have first been cloned in 2000. Since “absorption” of fetuses is relatively common in domestic pigs, it would be of interest to know whether these “resorbed” fetuses have chromosomal errors. This is especially the case because a variety of chromosomal errors have been identified in domestic pigs.
Acknowledgement
I appreciate very much the help of the pathologists at the San Diego Zoo.
References
Berger, E.M., Leus, K. and Schwarzenberger, F.: Faecal steroid metabolites for non-invasive assessment of reproduction in common warthogs ( Phacochoerus africanus ), red river hogs ( Potamochoerus porcus ) and babirusa ( Babyrousa babyrussa celebensis ). Pp. 411-412, In, Proceed. Europ. Assoc. Zoo- and Wildlife Veterinarians, Heidelberg , May 8-12, 2002 .
Boomker, J.: Parasites of South African wildlife. V.A. description of the males of Oesophagostomum mocambiquei Ortlepp, 1964 from warthogs, Phacochoerus aethiopicus (Pallas, 1766). Onderstepoort J. Vet. Res. 57:169-173, 1990.
Bosma, A.A.: The chromosomal G-banding pattern in the wart hog, Phaecochoerus aethiopicus (Suidae, Mammalia) and its implications for the systematic position of the species. Genetica 49:15-19, 1978.
Bosma, AA., deHaan, N.A. and MacDonald, A.A.: The current status of cytogenetics of the Suidae: A review. Bongo 18:258-272, 1991.
Claxton, J.R., Faye, J.A. and Rawlings, P.: Trypanosome infection in warthogs ( Phacochoerus aethiopicus ) in the Gambia . Vet. Parasitol. 41:179-187, 1992.
Eckstein, P. and Zuckerman, S.: The oestrus cycle in the mammalia. Chapter 4 (pp. 226-397), in Marshall 's Physiology of Reproduction, 3 rd ed. A.S. Parkes, Ed. Longmans, Green and Co., London , 1956.
Geisert, R.D.: Pigs. Pp. 792-799 in Vol. III of, Encyclopedia of Reproduction, E. Knobil and J.D. Neill, eds., Academic Press, San Diego, 1998.
Groves , C.: Ancestors for the pigs: taxonomy and phylogeny of the genus Sus . Technical Bulletin No. 3. Department of Prehistory, Research School of Pacific Studies, Australian National University , 1981.
Horak, I.G., Biggs, H.C., Hanssen, T.S. and Hanssen, R.E.: The prevalence of helminth and arthropod parasites of warthog, Phacochoerus aethiopicus , in South West Africa/Namibia. Onderstepoort J. Vet. Res. 50:145-148, 1983.
Jones, M.L.: Longevity of ungulates in captivity. Int. Zoo Yearbk. 32:159-169, 1993.
Keffen, R.H., van Heerden, J., Dauth, J. and Dreyer, M.J.: Blood chemical parameters in the warthog Phacochoerus aethiopicus . J. S. Afr. Vet. Ass. 58:137-142, 1987.
Kenny, D.E., Braselton, W.E., Taylor , R.A., Morgan, T. and Hesky, R.B.: A case of anaemia in a neonatal warthog ( Phacochoerus aethiopicus ) and evaluation of serum-soluble iron in warthogs. J. S. Afr. Vet. Ass. 73:139-141, 2002.
Macdonald, A.A. and Bosma, A.A.: Notes on placentation in the Suina. Placenta 6:83-91, 1985.
Marczynska, B. and Pigon, H.: Somatic chromosomes of the native African pig. Cytologia 38:111-116, 1973.
Melander, Y. and Hansen-Melander, E.: Chromosome studies in African wild pigs (Suidae, Mammalia). Hereditas 92:283-289, 1980.
Mentis, M.T.: A review of some life history features of the large herbivores of Africa . The Lammergeyer 16 June, 1-89, 1972.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Oura , C.A. , Powell, P.P. and Parkhouse, R.M.: Detection of African swine fever virus in infected pig tissues by immunocytochemistry and in situ hybridization. J. Virol. Methods 72:205-217, 1998.
Patten, B.M.: The Embryology of the Pig. 2 nd ed. The Blakiston Co. Philadelphia, 1931 (reprinted 1947).
Ramsey, E. M.: The Placenta. Human and Animal. Praeger , N.Y. , 1982.
Rothschild, M.F. and Ruvinsky, A., eds.: The Genetics of the Pig. Oxford University Press, 1998.
Stolte, M., Odening, K., Quandt, S., Bengis, R.G. and Bockhardt, I. : Sarcocystis dubella n. sp. and Sarcocystis phacochoeris n. sp. (Protozoa: Sarcocystidae) from the warthog ( Phacochoerus aethiopicus ) in South Africa . J. Eukarot. Microbiol. 45:101-104, 1998.
Thenius, E.: Zur Evolution und Verbreitungsgeschichte der Suidae (Artiodactyla, Mammalia). Z. Säugetierk. 35:321-342, 1970.
Wallace, C. and Fairall, N.: The chromosomes of the warthog. S. Afr. J. Med. Sci. 32:51-54, 1967.
Watanabe, T., Hayashi, Y., Ogasawara, N. and Tomoita, T.: Polymorphism of mitochondrial DNA in pigs based on restriction endonuclease cleavage patterns. Biochem. Genet. 23:105-113, 1985.
Woodall, P.F.: Periodontal disease in southern African bushpigs ( Potamochoerus porcus ) and warthogs ( Phacochoerus aethiopicus ). J. Wildl. Dis. 25:66-69, 1989.