| January9 |
Lagostomus maximus
Order: Rodentia
Family: Chinchillidae
1) General Zoological Data
The viscacha belongs to the South American group of “ hystricomorpha ”, animals defined by an especially large infraorbital enlargement for the descent of the masseter muscle. The South American rodents are unique in many ways; they had their origin in the African Eocene and perhaps (? presumably) rafted to South America when the continents split. But there is considerable argument about this point and, especially, whether the African porcupines should be included in this possibly coherent, paleontologic assemblage. George Gaylord Simpson (1974), in the introductory remarks to the hystricomorph symposium in London that considered these details, made some very cogent observations and suggested that we should preferably use the term “ Caviomorpha ” when referring to the animals there discussed. This is an interesting and yet to be resolved issue where molecular means may ultimately become the most useful tools for providing a definitive answer (see Nedbal et al., 1995).
There are mountain viscachas and plains viscachas. They are related but differ in chromosome number and a number of other features. The animal whose placenta is described here is the plains viscacha from Argentina . This large South American rodent lives in Argentina and southern Paraguay . It is a crepuscular animal that weighs between 2 and 4.5 kg (females) and 5-8 kg (for the aggressive males) (Weir, 1974). It inhabits deep burrows (“ viscacheras ”) that have numerous large entrances. Their longevity in captivity is 9 years 5 months (Weir, 1971a). The animals are considered a pest by farmers and are widely hunted which has severely reduced their numbers. Many similarities to chinchillas exist (see that chapter). Whether chinchillas really “belong” to the hystricomorpha is also debated, and the reader is referred to the comprehensive symposium by Rowlands & Weir (1974) and the discussion already detailed in my chapters on Pacarana and Chinchilla, as well as in Simpson's introductory remarks. Moreover, newer studies of mitochondrial RNA genes by Nedbal et al. (1995) suggested that there was a split in Eocene between Old and New World rodent groups and that the caviomorphs are indeed a monophyletic group. Thus, ongoing debate attempts to better understand the variety of South American rodents and their origin as well as taxonomic relations.
In their very detailed study of the chromosomes of all ‘hystricomorphs', including those of the viscacha, George & Weir (1974) specifically discussed the potential evolutionary relationships of these rodents which, as far as chromosomes are concerned, whose speciation was accomplished mostly by Robertsonian fusions. In discussing one of the papers of that symposium on hystricomorphs, G.G. Simpson drew further attention to the as yet unknown ultimate origin and time of arrival of all of the South American rodents. Walker et al. (2000) characterized microsatellite loci of mountain and plains viscacha. They found that of the ten primers from mountain viscacha, eight amplified a band of appropriate size in the mountain viscacha sample, four did for the plains viscacha, and some identified loci in chinchilla samples.
 |
Plains viscacha (Courtesy Johns Hopkins Press; from Nowak, 1999). |
2) General Gestational Data
The length of gestation averages 145-166 days, with usually 2 (1-4) offspring produced. These weigh on average 200 g. Viscachas have unusual reproductive parameters: between 300-800 ova are produced at each cycle (Mossman & Duke, 1973), about 10% are fertilized, but only 2 (1-4) offspring result.
3) Implantation
Implantation of the viscacha blastocyst occurs on day 18 and is antimesometrial (Roberts & Perry, 1973; 1974; Mossman, 1987). It is completely interstitial and was beautifully detailed in a publication by Roberts & Weir (1973). The viscacha placenta is in many respects very similar to that of the guinea pig, and also to that of some of the other South American rodents. It is discoid, hemo-monochorial, with a complex labyrinth, and it is “pedunculated”. It has a subplacenta and connects only through a narrow stalk with the decidualized endometrium. It forms an inverted yolk sac relationship to the endometrium, and possesses a thick layer of trophoblastic giant cells at the base that invade deeply into endo-myometrium (more detailed representation is made in the chapter on chinchilla that should be consulted). Indeed, the structure of the viscacha placenta is so similar to that of the chinchilla that it is unnecessary to repeat the detailed description here. As indicated earlier, implantation occurs 18 days after copulation which is much longer than in the guinea pig (3 days) and other hystricomorphs (Roberts & Perry, 1973; 1974).
4) General Characterization of the Placenta
As the chinchilla placenta, this viscacha placenta is equally similar to that of the guinea pig, as well as to those of other South American rodents. It is a discoid hemo-monochorial organ, with a complex labyrinth, and it is also "pedunculated “, with a stalk. It has a subplacenta and connects only through the narrow stalk with the decidualized endometrium. It forms an inverted yolk sac relationship to the endometrium and has a thick layer of trophoblastic giant cells at the base that invade deeply into endo-myometrium. For the novice, not familiar with inverted yolk sac placentation, this is a complex configuration with the development of several sites for nutrient exchange. Reference is made to the chapters on guinea pig, chinchilla, and pacarana for additional details and diagrams. In most respects these placentas have all the same morphology. The vascular anatomy of hystricomorph rodents has been beautifully studied and pictured by Miglino et al. (2004).
The placenta shown here comes from a pregnant female near term that died from infection. The dam weighed 4.74 kg; the male fetus weighed 244 g and had a 19 cm CR length. The placenta weighed 39.5 g and measured 6 x 4.5 x 2.5 cm; it had a 10 cm umbilical cord.
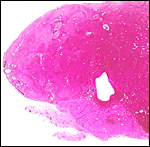 |
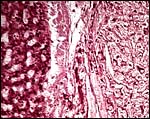
Macroscopic view of one-half of this viscacha placenta showing the broad subplacenta (pale) at the base. |
Since this summary a decisive paper has been published by Flamini et al. (2011). It describes the details of implantation into the uterus of six 40-60 day gestations, five mid-term pregnancies, and four full term gestations and it also includes the picture of a mid-term implanted placenta with fetus.
5) Details of fetal/maternal barrier
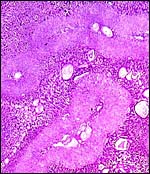
This is a labyrinthine, hemo-monochorial placenta according to Enders' (1965) definition that has, in addition to the syncytial rings of the lobules, spongy zones, as is shown in the photographs. The more detailed aspects and comparisons with other hystricomorph placentas need to be read in the contributions by Roberts & Perry (1973, 1974) and the other rodents I have described in some detail.
6) Umbilical cord
The cord is divided into the portion supplying the inverted yolk sac, and the embryonic allantoic cord, as is the case in all rodents. Both are very short but measurements are not generally available, except in this gestation; and here the cord was 10 cm long. Regrettably, my sections did not include the cord, thus I can only presume that each portion contains two vessels, as is the case in the pacarana (see that chapter).
7) Uteroplacental circulation
I do not have appropriate specimens to study this aspect of reproduction in viscachas but it is assumed that the vasculature is very similar to that of the guinea pig whose chapter should be consulted, in addition to the fine study of the vascular arrangement within the placenta undertaken by Miglino et al. (2004).
8) Extraplacental membranes
Amniogenesis is by cavitation, not by folding, and it occurs 28 days post coitum; the allantois develops 7 days later (Roberts & Perry, 1974). Typical amnionic “pustules” (loose connective tissue) are there also depicted. In my placenta, no “pustules” were identified. The amnion was smooth.
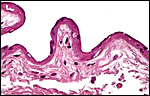 |
Amnion of the present placenta. |
9) Trophoblast external to barrier
There is trophoblastic infiltration (giant cells) into the “subplacenta”, a region of degenerated material and clots. See the chapters on other South American rodents in which this is more fully delineated.
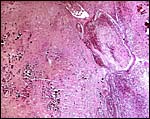 |
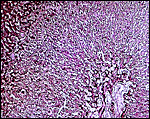
Amnion of the present placenta. |
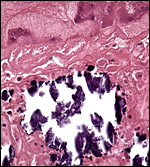 |
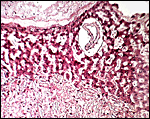
Higher magnification of the subplacenta (below) as it borders (above) the giant cell layer. The necrosis and calcifications are typical and normal. |
10) Endometrium
The decidual transformation of rodent gestations, including the reduction of the decidual thickness at the antimesometrial pole, has been summarized in great detail in the study of Abrahamsohn & Zorn (1993). There is decidual transformation of the endometrium. In addition, one may identify some granulated metrial cells scattered throughout that have been discussed for rodent uteri in some detail by Peel (1989). She also referred to the rich and somewhat confusing literature on this topic. The then current view (for rats and mice) was that these cells are specialized “killer cells” that target trophoblast.
11) Various features
This species, as is true of many other South American rodents, has a subplacenta. While it had initially been speculated that the “subplacenta” was a feature exhibited only by caviomorph rodents, subsequent studies have shown it to be present also in bathyergids, hystricids and thryonomids, according to Luckett (1980; see also Roberts & Perry, 1974). This region is thought to be a “growth zone for the proliferation of the trophoblast in the lobulated placental disc, and it has also been suggested as a possible site of placental endocrine activity”. Apparently, the degenerative changes in it occur only a few days before parturition. The finding of PAS-positive cells by Amoroso (in discussion) and the notion that this site is responsible for PBG synthesis (Heap & Illingworth, 1974) may partially explain the unusual nature of this region. Maternal blood flow in these lacunae ceases already few weeks post conception. Fetal capillarization of the subplacenta starts only thereafter. Consequently, the subplacenta in no stage of pregnancy is simultaneously supplied by both, maternal and fetal blood flow and therefore cannot serve as an exchange organ. It degenerates a few days prior to parturition and leaves a mass of cellular detritus at the later site of placental separation.
In addition, the narrow “stalk” of the attachment site is characteristic of these rodents' placentas and it becomes quite attenuated at term gestation. This stalk comes about, as Luckett stated, by: “the fibrovascular ring which surrounds the site of attachment of the yolk sac splanchnopleure to the mesodermal body stalk at the fetal surface of the placenta. We have also observed an identical fibrovascular ring in hystricids and bathyergids. The functional significance of this structure remains unclear at present” (Luckett, 1980).
The ovaries of viscachas are as unusual as those of the pacarana; perhaps they are even more lobulated and filled with developing follicles. Mossman & Duke (1973) have several photographs of these structures that come from the extensive studies conducted by Barbara Weir in London (Weir & Rowlands, 1974). The uterus, of course, is bicornuate, and the upper portions of the vagina (and the remarkably amuscular cervix of course) are septate (Weir, 1971c).
The embryological development of various hystricomorphs, including that of the viscacha, is detailed by Roberts & Perry (1973; 1974). They make the point that resorption of embryos characteristically occurs in viscacha gestations. Weir (1971b) had earlier indicated that those embryos that are implanted nearest the cervix survived, the others became resorbed.
12) Endocrinology
The estrous cycle is approximately 45 days long. Gadsby et al. (1980) looked for trophoblastic aromatase activity in a variety of species, but did not find estrogenic evidence for it in viscachas. The corpus luteum persists throughout gestation and appears to be necessary for the maintenance of pregnancy (Weir, 1971c). Tam (1974) recorded the progesterone metabolism of several hystricomorph rodents, especially that of the chinchilla. Some animals depend on the production of progesterone from the accessory corpora lutea, and a dependency on progesterone-binding protein was suggested. Apparently all have the capacity eventually to produce this hormone from the syncytiotrophoblast of the spongy zone in the placenta; but, the viscacha was not studied. Tam also affirmed that the hydroxyl dehydrogenase is present in the large quantity of interstitial tissue of the ovaries, not only in the corpora lutea and the luteinized accessory follicles. That region is absent in the guinea pig and thus it does not contribute to progesterone production; it probably is mainly productive of androgens and estrogens. Heap & Illingworth (1974), in the same volume, studied the contribution of the progesterone-binding globulin (PBG) to the maintenance of pregnancy. It was found in several hystricomorphs, including the viscacha, and its presence was dependent on a viable pregnancy. The authors suggested that it was probably secreted in the unique subplacenta of the hystricomorphs; it was absent from the fetal circulation.
13) Genetics
The plains viscacha has 56 chromosomes (in contrast to chinchilla and mountain viscacha which have 64 chromosomes). Nearly all chromosomes are metacentrics, and one pair has a pronounced and typical secondary constriction (Wurster et al., 1971). The karyotypes shown below are from Hsu & Benirschke (1971). A very detailed study of the chromosomes of all hystricomorphs, including those of the viscacha, was published by George & Weir (1974). That report was especially concerned with the potential evolutionary relationships (mostly Robertsonian fusions) of these rodents. Walker et al. (2000) characterized microsatellite loci of mountain and plains viscachas (see first paragraph). Graphodatskaya et al. (2001) have confirmed the karyotypic findings in a viscacha colony at Zürich Zoo and they did Giemsa banding for the first time. The Argentinian population they studied had only metacentrics or submetacentric chromosomes, while the Zürich animals had one pair of acrocentrics, similar to the female karyotype shown below.
 |
Chromosomes of male and female viscachas (from Hsu & Benirschke, 1971). |
14) Immunology
I am not aware of any such studies in viscacha. I assume that immunoglobulin transfer from mother to fetus occurs via the inverted yolk sac epithelium, as is typical of rodents.
15) Pathological features
Few data are available on the pathological features found in this species. Couch et al. (2001) identified that 19% of fecal samples collected from colonies of viscacha in Argentina contained coccidian organisms, all of the genus Eimeria, with two new species described. Laciar et al. (1994) identified (by a complex methodology) Listeria monocytogenes in viscacha. In the Zürich zoo, diabetes with lens opacification was reported by Graphodatskaya et al. (2001). Weir (1971c) described that deaths occurred because of fighting and that this had led to infections with Salmonella typhimurium and Pasteurella pseudotuberculosis.
16) Physiologic data
Goode et al. (1981) described the composition of viscacha milk in some detail. Numerous studies have been undertaken to understand the role of the viscacha pineal gland; many other reports define male reproductive parameters. Weir, who advocated this species as laboratory animal (1971b) provided information on the method of anesthesia; halothane was very effective and the animals recovered quickly. She also made a detailed study of the reproductive organs of female viscachas (1971c). That report shows impressively the bicornuate nature of the upper vagina, cervix and uterus. The cervix is remarkably amuscular and a squamous septum forms over the cervical opening that sloughs at ovulation.
17) Other resources
We have no specimens of viscacha cells in the CRES cell bank at San Diego Zoo.
18) Other remarks – What additional Information is needed?
More details of the size of the placenta, length of the umbilical cord, endocrinology, and early implantation specimens are needed.
Acknowledgement
The animal photograph in this chapter comes from the files of the Johns Hopkins Press and is the same as shown in Nowak (1999) and was taken by Walker.
References
Abrahamsohn, P.A. and Zorn, T.M.T.: Implantation and decidualization in rodents. J. Exp. Zool. 266:603-628, 1998.
Couch, L., Foster, G.W., Machicote, M. and Branch, L.C.: Description of two new species of Eimeria (Apicomplexa: Eimeriidae) and Eimeria chinchillae -like oocysts from the plains viscacha Lagostomus maximus (Desmarest, 1817) (Rodentia: Chinchillidae) from Argentina . J. Parasitol. 87:144-147, 2001.
Enders, A.: A comparative study of the fine structure of the trophoblast in several haemochorial placentas. Amer. J. Anat. 116:29-68, 1965.
Flamini, M.A., Portiansky, E.L., Favaron, P.O., Martins, D.S., Ambrósio, C.E., Mess, A.M., Miglino, M.A. and Barbeito, C.G.: Chorioallantoic and yolk sac placentation in the plains viscacha (Lagostomus maximus) – A caviomorph rodent with natural polyovulation. Placenta 32:963-968, 2011.
Gadsby, J.E., Heap, R.B. and Burton , R.D.: Oestrogen production by blastocyst and the early embryonic tissue of various species. J. Reprod. Fertil. 60:409-417, 1980.
George, W. and Weir, B.J.: Hystricomorph chromosomes. Sympos. Zool. Soc. London 34:79-108, 1974. Academic Press, London, 1974.
Goode, J.A., Peaker, M. and Weir, B.J.: Milk composition in the plains viscacha (Lagostomus maximus). J. Reprod. Fertil. 52:563-566, 1981.
Graphodatskaya, D., Wenker, C., Steiger, D. and Stranzinger, G.: Two different karyotypes in two plains viscacha ( Lagostomus maximus ) populations. Proceed. 12 th Amer. Colloquium on Animal Cytogenetics and Gene Mapping, Davis , CA. p. 11, 2001.
Heap, R.B. and Illingworth, D.V.: The maintenance of gestation in the guinea-pig and other hystricomorph rodents: Changes in the dynamics of progesterone metabolism and the occurrence of progesterone-binding globulin (PBG). Sympos. Zool. Soc. London 34:385-415, 1974. Academic Press, London , 1974.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 6; Folio 281, 1971. Springer-Verlag , New York .
Laciar, A.L., Picca, S. and de Centorbi, O.P.: Isolation of Listeria seeligery from cecum of viscacha (Lagostomus maximus maximus). Rev. Argent. Microbiol. 26:183-188, 1994. (In Spanish).
Luckett, W.P.: Monophyletic or diphyletic origins of anthropoidea and hystricognathi. Evidence of the fetal membranes. Chapter 17, pp. 347-368, in: Evolutionary Biology of the New World Monkeys and Continental Drift. R.C. Ciochon & A.B. Chiarelli, Eds. Plenum press, N.Y., 1980.
Miglino, M.A., Carter, A.M., Ambrosio, C.E., Bonatelli, M., de Oliveira, M.F., dos San Ferraz, R.H., Rodriguez, R.F. and Santos, T.C.: Vascular organization of the hystricomorph placenta: a comparative study in the Agouti, Capybara, Guinea Pig, Paca and Rock Cavy. Placenta 25:438-448, 2004.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison , Wisconsin , 1973.
Nedbal, M.A., Allard, M.W. and Honeycutt, R.L.: Molecular systematics of hystricognath rodents: evidence from the mitochondrial 12S rRNA gene. Mol. Phylogenet. Evol. 3:206-220, 1994.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Peel, S.: Granulated metrial cells. Advances Anat. Embryol. Cell Biol. 115:1-112, 1989.
Roberts, C.M. and Perry, J.S.: Hystricomorph biology and embryology, pp.333-360, in: The Biology of Hystricomorph Rodents. I.W. Rowlands and B.J. Weir, eds. Sympos. Zool. Soc. London, # 34. Academic Press, London , 1974.
Roberts, C.M. and Weir, B.J.: Implantation in the plains viscacha, Lagostomus maximus . J. Reprod. Fertil. 33:299-307, 1973.
Rowlands, I.W. and Weir, B.J.: The Biology of Hystricomorph Rodents. Sympos. Zool. Soc. London, # 34. Academic Press, London, 1974.
Simpson, G.G.: Chairman's introduction: Taxonomy. In: The Biology of Hystricomorph Rodents. Sympos. Zool. Soc. London, # 34. Pp.1-5. Academic Press, London , 1974.
Smith, H.A., Jones, T.C. and Hunt, R.D.: Veterinary Pathology. Lea & Febiger, Philadelphia , 1972.
Tam, W.H.: The synthesis of progesterone in some hystricomorph rodents. Sympos. Zool. Soc. London. 34:363-384, 1974. Academic Press, London , 1974.
Vidal, O.R., Riva, R. and Spirito, S. The chromosome of the South American rodent vizcacha (Lagostomus maximus). Caryologia 26:77, 1973.
Walker , R.S., Farmerie, W.G. and Branch, L.C.: Characterization of microsatellite loci from the mountain vizcacha Lagidium viscacia and their use for the plains vizcacha Lagostomus maximus . Mol. Ecol. 9:1672-1674, 2000.
Weir, B.J.: The reproductive physiology of the plains viscacha, Lagostomus maximus . J. Reprod. Fertil. 25:355-363, 1971a.
Weir: The plains viscacha as a laboratory animal. J. Physiol. 215:2p-4p, 1971b.
Weir, B.J.: The reproductive organs of the female plains viscacha, Lagostomus maximus . J. Reprod. Fertil. 25:365-373, 1971c.
Weir, B. J.: Reproductive characteristics of hystricomorph rodents. Sympos. Zool. Soc. London 34:265-301, 1974. Academic Press, London , 1974.
Weir, B. J. and Rowlands, I.W.: Functional anatomy of the hystricomorph ovary. Sympos. Zool. Soc. London 34:303-332, 1974. Academic Press, London , 1974.
Wurster, D.H., Snapper, J.R. and Benirschke, K.: Unusually large sex chromosomes: new methods of measuring and description of karyotypes of six rodents ( Myomorpha and Hystricomorpha) and one lagomorph (Ochotonidae). Cytogenetics 10 :153-176, 1971.