| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Oct 9, 2008. |
Desmodus rotundus murinus
by
Jana Ritter1, Rebecca Smedley1 and Kurt Benirschke
(Diagnostic Center for Population and Animal Health, Michigan University, Lansing, MI1)
Order: Chiroptera
Family: Desmodontidae (Phyllostomidae)
1) General Zoological Data
The uterus with placenta, and other details were made available by Dr. Dalen Agnew, Lansing, MI and the specimen came from a private collector and originated from the Milwaukee County Zoo. Inquiries to the ‘Organization for Bat Conservation’ (www.batconservation.org) verified this information. It is presumed that this animal died from sepsis but, histologically, there was no inflammation; the intestines were filled with recently ingested blood.
This family (Desmodontidae) has recently been assigned to being a subfamily (Desmondontinae) according to Wilson & Reeder (1993); it consists of three or four members (formerly 8 species were recognized). These are South American bats with the characteristic specialization of sucking blood (20 ml every night per animal) from cattle, horses, pigs etc. and usually having pointed ears. Adults weigh 15-50 g with the single newborns weighing around 5-7 g (Hayssen et al., 1993). Maximal longevity (in captivity) of 29 years was listed by Weigl (2005); others have suggested that the longevity in nature is more like 9 years. A number of captive colonies exist and they are relatively easily kept. The range of vampire bats is perhaps expanding; at least they are not an endangered species. In contrast to other bat species, vampires do not hibernate and they are said to die within 2 days when no blood has become available. Thus, sharing of blood in colonies has been observed. Although the vampire bats differ from many other bat species, they are nocturnal and also employ echolocation. A comprehensive review of this species has been produced by Greenhall et al. (1983). The Latin name comes from desmos (Gr. bundle) and odous (Gr. from ondontous) because of their ‘bundled’ cone-shaped upper central incisors (Gotch, 1979).
Desmodontidae are close relatives of Phyllostomidae (even though the latter have markedly different chromosome numbers), as is expressed in various classifications. Moreover, Wimsatt (1954) makes this connection in his extensive review of early vampire bat implantation. He described the presence of a unicornuate uterus in phyllostomid bats, while vampire bats have a bicornuate uterus.
 |
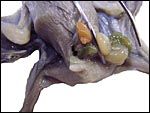
The pregnant vampire bat under discussion. |
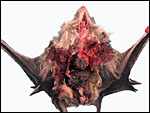 |
Opened abdomen of this bat. |
 |
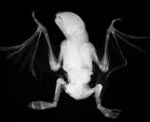
Vampire bat in flight. |
 |
Vampire bat from Web Site of National Geographics. |
2) General Gestational Data
The length of gestation is listed as ~205+ days (Hayssen et al., 1993). The initial implantation site is antimesometrial (Mossman, 1987) and it is also ‘interstitial’ in decidua. The term placenta is discoid with a complex labyrinth, a basal trophospongium and it is said to be at first endotheliochorial in nature but then becoming hemodichorial. After exhaustive exploration of the early stages of placental development, Wimsatt (1954) still considered the placenta to have an endotheliochorial character although most bats are hemochorial. In later electronmicroscopic studies he made it to be hemochorial again (Björkman & Wimsatt, 1968). Wimsatt & Trapido (1952) delineated the reproductive physiology of ovary and testis in this polyestrous species from numerous specimens obtained in Panama. The placental weight at term had not been determined. In the following specimen of a term fetus the weight was 0.6 g after formalin fixation.
3) Implantation
Early implantational stage have not been observed by us but Mossman (1987) described them to be antimesometrial and interstitial; details have been elaborated upon by Wimsatt (1954). A permanent bilaminar omphalopleure develops from incomplete inversion of the yolk sac that becomes also periplacental; this will be shown below. An early gestation has been described by Quintero & Rasweiler (1974).
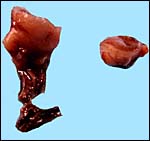
In August 2008 a term fetus with attached placenta was kindly made available by Natalie Lindholm of the Dallas World Aquarium. This appears to have been term gestation; the male fetus with placenta weighed (after formalin fixation) 9.2 g, had a 5 cm CR length, and its very thin umbilical cord was 3 cm long. The placenta measured 0.9 x -.3 cm and weighed 0.6 g. It had a central insertion of the umbilical cord. The next two photographs show the ventral and dorsal sides of this neonate and placenta. The abdomen appears to be disrupted by an omphalocele through which some intestines and the spleen extrude. A small portion of membranes (?omphalocele sac) accompanied the specimen.
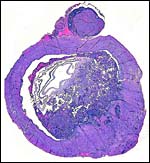
4) General Characterization of the Placenta
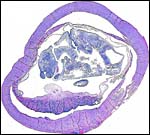
This is a discoid hemochorial placenta with a larger labyrinth and a smaller and variably thick trophospongium composed of large cytotrophoblastic cells at the site of interstitial implantation. There is deep infiltration by trophoblastic cells that extend into the parametrial vascular adventitia.
5) Details of fetal/maternal barrier
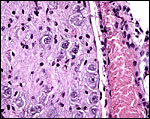
At this stage of development, this is clearly a hemochorial (hemodichorial) placenta in the labyrinthine region with the trophoblast being double-layered. Mitoses were not observed in the cytotrophoblast and it is not certain from observations at this stage that the outer layer of trophoblast is truly syncytial, as was seen in the EM study by Björkman & Wimsatt (1968). It is of historical interest that Wimsatt changed his mind several times between hemochorial and endotheliochorial types of implantation, depending on the stages and staining methods. The EM study just referred to appears to settle the point that hemochorial is the end stage while initially there is a maternal endothelial interface. The cytotrophoblast is separated from the fetal blood by a basement membrane in which tiny transport vesicles seen electronmicroscopically suggest an exchange function. No truly microvillous surface is seen on the syncytiotrophoblast although a syncytium appears to be present in the EM study. The fetal capillaries are scattered through a nondescript connective tissue villous core and are distant to the trophoblastic surface. Typical Hofbauer cells were not observed. At the stage of development we observed here, there were no remains of a maternal endothelium or of the maternal vascular “tubes” that can apparently be observed at earlier stages. In any event, the structure is much more villous than had been suggested earlier.
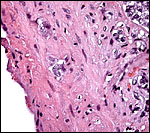
The spongiotrophoblastic region is a much thinner than the labyrinth and it is an irregularly thick layer at the placental base extending with ‘fingers’ into the myometrium around maternal vessels. Its cells are very deeply stained, have much larger nuclei, and they possessed an usually vacuolated cytoplasm. As they extend into the myometrium, the more peripheral cells are often multinucleated and have a much more homogeneous eosinophilic cytoplasm. Vascular invasion by trophoblast was not observed, nor was there a typical decidua at the site of implantation.
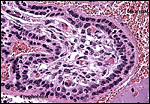 |
Villous surface with double-layered trophoblast. (FC=fetal capillary). |
6) Umbilical cord
The length of the umbilical cord was not measured in this specimen but Wimsatt (1951) stated that it is 21 mm long at term with a single twist. The sections of the immature specimen shown here neither allow a determination of the number of blood vessels nor the possible presence of any ducts. Wimsatt (1954), however, has depicted the content of the cord with two allantoic arteries, one vein and single vitelline artery and vein. In contrast, the umbilical cord of the term pregnancy discussed here was extremely thin. A segment had to be suspended in liver tissue for adequate cross sections to be obtained. It measured 3 cm in length and had two arteries, two veins and an allantoic duct. There were no twists and there also was virtually no Wharton’s jelly.
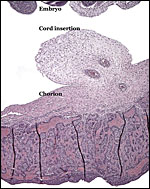 |
Placental surface with insertion site of the umbilical cord. |
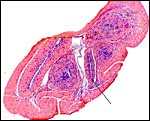 |
Umbilical cord of term placenta with duct at arrow. |
7) Uteroplacental circulation
This has not been studied so far as we can determine but the infiltration of maternal arteries through the trophospongium has been shown several times.
8) Extraplacental membranes
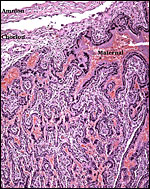
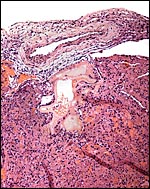
The allantois is rudimentary (Mossman, 1987); it was also not evident in these sections or in any of Wimsatt’s specimens. The amnionic epithelium is very thin, in contrast to the thicker, avascular connective tissue of this membrane whose ultimate origin is in dispute (Wimsatt, 1954). It is difficult to state with assurity whether this membrane represents some of Reichert’s membrane as is discussed in detail by Wimsatt (1954). The ‘free’ membranes otherwise consist largely of an extensive bilaminar omphalopleure. While a typical decidua capsularis exists in many bats, it is not apparent in these sections and Wimsatt (1954) also described it as becoming progressively attenuated.
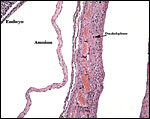 |
Free membranes with uterus at right and embryo at left. |
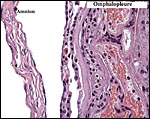 |
Higher magnification of free membranes. |
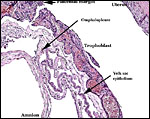 |
This is from the edge of the placenta with the origin of the membranes. |
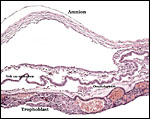 |
Similar view as previous slide. |
9) Trophoblast external to placenta and in uterus
There is rather marked and deep trophoblast infiltration as shown in several sections below. While this extraplacental trophoblast occurs in clusters and is located deep, beneath the implanted placenta (but not on the opposite side), it does not appear to occur within maternal blood vessels. Whenever it is close to the vessel, an endothelial sheet of tissue separates the trophoblast from the maternal blood. These infiltrating trophoblastic cells are large, somewhat vacuolated cells with large irregular nuclei. Similar trophoblast is also present next to the periovarian blood vessels. It is thus not clear in what type of space these cells propagate and it is remarkable that this deep infiltration has not been observed by Wimsatt.
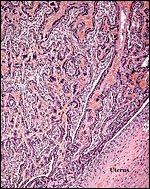
10) Endometrium
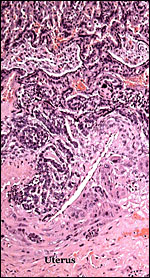
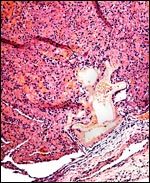
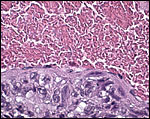
Vampire bats have a bicornuate uterus in contrast to the phyllostomidae and, from the observations recorded here (see the first photomicrograph), they possess an ovarian bursa. Wimsatt (1954) also described the presence of an ovarian bursa while Mossman & Duke (1973) listed in their comprehensive studies that no ovarian bursa is found in the Desmodontidae. Wimsatt (1954) and Mossman (1987) described the development of a large amount of decidua in vampire bats, particularly in the vicinity of implantation. This was not obvious in the early specimen shown here and since much “necrolysis” takes place in this region, the absence in this specimen may not be surprising. Elsewhere in the uterus (e.g. the parietalis) decidua does not form as is seen in the next photomicrograph.
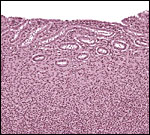 |
Endometrium in this pregnant uterus – this does not appear to be a decidua. |
11) Various features
Vampire bats, as many other bats, have a vomeronasal organ that, in these species, may have a special role for their complex feeding behavior (Cooper & Bhatnagar, 1976). Much attention has been directed to the transmission of rabies by vampire bats and for that reason various ‘control’ systems have been devised. More importantly perhaps is the isolation from their saliva of an anticoagulant that is widely used for the experimental treatment of strokes etc. Also, because of this feature, several EM studies of the salivary glands are recorded in the literature; they can be accessed through PubMed.
12) Endocrinology
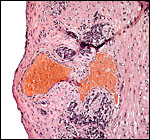
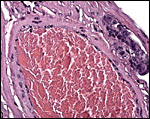
Many studies have directed their attention to this ‘control’ of vampire bats in part because of their ability to transmit rabies (see Scheffer et al., 2007). Thus, Serrano et al. (2007) fed bovine blood containing coumestrol that was found to affect ovaries and testes; others used various toxins or toxic anticoagulants in cattle for ‘bat control’ (Thompson et al. 1972). The exemplary description by Wimsatt & Trapido (1952) concerns the ovarian development of oocytes and corpora lutea in bats of different developmental stages; it also considers the testicular development. The vacuolation of luteal cells suggested lipid content. They observed two CLs only once but no twins were found.
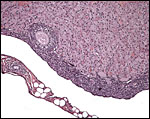 |
Corpus luteum of pregnancy with adjacent developing follicle; note the bursa. |
13) Genetics
The vampire bat has 28 chromosomes as is detailed in several studies (Hsu & Benirschke, 1967; Yonenaga et al., 1969; Capanna et al., 1970; Varella-García et al., 1989; O’Brien et al., 2006). Santos et al. (2001) have done undertaken special staining procedures and found C-banding in all autosomal centromeres; they commented that the sex chromosomes are entirely heterochromatic. The NOR was located on chromosome # 8. To our knowledge, no hybrids have been identified.
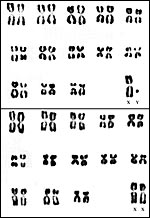 |
Karyotype from Hsu & Benirschke, 1967. |
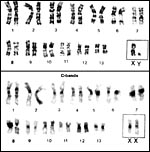 |
Karyotypes from O’Brien et al., 2006. |
14) Immunology
We are not aware of any studies other than those concerning rabies virus.
15) Pathological features
The carriage of various virus antibodies, in different bat species, has been described by Price (1978). In a study on enteric bacteria of different bat species, Pinus & Müller (1980) found that Desmodus contains often pure cultures of Aeromonas hydrophila; at times they were admixed with other organisms. Gettinger & Gribel (1989) and in another study, Azevedo et al. (2002) described the mites of various bats, including Desmodus.
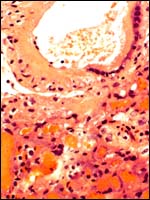
Because of the enormous iron intake by vampire bats, it is not surprising that some iron is deposited in the intestinal epithelium (but apparently not in the duodenum), less so in the liver, minimally in renal tubules adjacent to blood vessels, and we found none in the sections of pancreas, uterus or periovarian tissue. An atretic ovarian follicle though had hemosiderin deposits. Iron deposition in vampire bats has been studied by Morton & Wimsatt (1980) with labeled iron and also electronmicroscopically. Later, Morton & Janning (1982) determined the iron turnover with labeled iron isotopes. Fecal iron excretion was found, and generally there was marked restraint on iron absorption.
Rabies virus has been identified in vampire bats but no disease; on the other hand, equine trypanosomiasis is also being transmitted and can be lethal to the bat (Wimsatt & Trapido (1952), while Clark (1948) reported recovery from the disease.
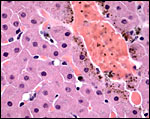 |
Liver with mild periportal iron deposit in hepatocytes. |
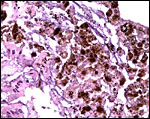 |
Surface of intestinal mucosa with numerous iron deposits in epithelium. |
16) Physiologic data
Normal blood values have been studied by Krutzsch & Wimsatt (1963). High red cell values and hemoglobin were found, hematocrits were also high, while WBCs were low. The saliva contains the anticoagulant ‘draculin’. The salivary plasminogen activators of bats are thrombolytic, affect fibrin in clots and they have been under consideration for the treatment of strokes (Wei et al., 2008). Numerous animal studies have been done to understand its mechanism (e.g. Witt et al., 1992; Grandjean et al., 2004; Dong et al., 2004).
We are not aware that significant other physiological studies such a blood flow measurements have been recorded.
17) Other resources
To the best of our knowledge, cell lines for study are not available; there are none at CRES.
18) Other remarks – What additional Information is needed?
We were unable to find any endocrinological information on vampire bats. Moreover, the absence of hemosiderin in the upper GI tract is puzzling.
Acknowledgement
The animal came to necropsy at Lansing, MI. The animal photographs in this chapter come from the Web Site of the National Geographics Society.
References
Azevedo, A.A., Marcos Linardi, M. and Coutinho Zanatta, M.T.: Acari ectoparasites of bats from Minas Gerais, Brazil. J. Med. Entomol. 39:553-555, 2002.
Björkman, N.H. and Wimsatt, W.A.: The allantoic placenta of the Vampire Bat (Desmodus rotundus murinus): A reinterpretation of its structure based on electron microscopic observations. Anat. Rec. 162:83-98, 1968.
Capanna, E. and Civitelli, M.V.: Chromosomal mechanisms in the evolution of chiropteran karyotype. Chromosomal table of Chiroptera. Caryologia 23:79, 1970.
Clark, H.C.: Equine trypanosomiasis – murina of Panama. Proc. 4th Intern. Congr. Trop. Med. and Malaria. 2:1342-1348, 1948.
Cooper, J.G. and Bhatnager, K.P.: Comparative anatomy of the vomeronasal organ complex in bats. J. Anat. 122(Pt.3):571-601, 1976.
Dong, N., Da Cunha, V., Citkowicz, A., Wu, F., Vincelette, J., Larsen, B., Wang, Y.X., Ruan, C., Dole, W.P., Morser, J., Wu, O. and Pan, J.: P-selectin-targeting of the fibrin selective thrombolytic Desmodus rotundus salivary plasminogen activator alpha1. Thromb. Haemost. 92:956-965, 2004.
Gettinger, D. and Gribel, R.: Spinturnicid mites (Gamasida: Spinturicidae) associated with bats in central Brazil. J. Med. Entomol. 26:491-493, 1989.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Grandjean, C., McMullen, P.C. and Newschwander, G.: Vampire bats yield clot buster for ischemic stroke. J. Cardiovasc. Nurs. 19:417-420, 2004.
Greenhall, A.M., Joermann, G., Schmidt, U. and Seidel, M.R.: Desmodus rotundus. Mammalian Species, No. 202, pp. 1-6, 1983.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell’s Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag, New York. Vol. 1, Folio 2, 1967.
Krutzsch, P.H. and Wimsatt, W.A.: Some normal values of peripheral blood in the vampire bat. J. Mammal. 44:556-559, 1963.
Morton, D. and Janning, J.T.: Iron balance in the common vampire bat Desmodus rotundus. Comp. Biochem. Physiol. A 73:421-425, 1982.
Morton, D. and Wimsatt, W.A.: Distribution of iron in the gastrointestinal tract of the common vampire bat: evidence for macrophage-linked iron clearance. Anat. Rec. 198:183-192, 1980.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Pinus, M. and Müller, H.E.: Enterobacteria of bats (Chiroptera). Zentralbl. Bakteriol. A 247:315-322, 1980 (in German).
Price, J.L.: Serological evidence of infection of Tacaribe virus and arboviruses in Trinidadian bats. Amer. J. Trop. Med. Hyg. 27:162-167, 1978.
O’Brien, S.J., Menninger, J.C. and Nash, W.G., eds.: Atlas of Mammalian Chromosomes. Wiley-Liss; A. John Wiley & Sons, Inc. Hoboken, N.J., 2006.
Quintero, H.F. and Rasweiler, J.J.: Ovulation and early embryonic development in the captive vampire bat, Desmodus rotundus. J. Reprod. Fertil. 41:265-273, 1974.
Santos, N., Fagundes, V., Yonenaga-Yassuda, Y and De Souza, M.J.: Comparative karyology of Brazilian bats Desmodus rotundus and Diphylla ecaudata (Phyllostomidae, Chiroptera): banding patterns, base-specific fluorochromes and FISH of ribosomal genes. Hereditas 134:189-194, 2001.
Scheffer, K.C., Carrieri, M.L., Albas, A., dos Santos, H.C., Kotait, I. and Ito, F.H.: Rabies virus in naturally infected bats in the state of São Paulo, Southeastern Brazil. Rev. Saudade Publ. 41:389-395, 2007 (in Portuguese).
Serrano, H., Pérez-Rivero, J.J., Aguilar-Setién, A., de-Paz, O. and Villa-Godoy, A.: Vampire bat reproductive control by a naturally occurring phytooestrogen. Reprod. Fertil. Dev. 19:470-472, 2007.
Thompson, R.D., Mitchell, G.C. and Burns, R.J.: Vampire bat control by systemic treatment of livestock with an anticoagulant. Science 177:806-808, 1972.
Varella-García, E., Morielle-Versute, M. and Taddei, V.A.: A survey of cytogenetic data on Brazilian bats. Rev. Brasil. Genet. 12:761-793, 1989.
Wei, Z., Wang, Y., Li, G., Li, X. and Liu, D.: Optimized gene synthesis, expression and purification of active salivary plasminogen activator alpha2 (DSPAalpha2) of Desmodus rotundus in Pichia pastoris. Protein Expr. Purif. 57:27-33, 2008.
Weigl, R.: Longevity of Mammals in Captivity; From the Living Collections of the World. [Kleine Senckenberg-Reihe 48]. E. Schweizerbart’sche Verlagsbuchhandlung (Nägele und Obermiller), Stuttgart, 2005.
Wilson, D.E. and Reeder, D.A.M.: Mammal Species of the World. A Taxonomic and Geographic Reference. 2nd ed. Smithsonian Institution Press, Washington, DC, 1993.
Wimsatt, W.A.: The fetal membranes and placentation of the tropical American Vampire bat Desmodus rotundus murinus with notes on the histochemistry of the placenta. Acta anat. 21:285-341, 1954.
Wimsatt, W.A. and Trapido, H.: Reproduction and the female reproductive cycle in the tropical American vampire bat, Desmodus rotundus murinus. Amer. J. Anat. 91:415-445, 1952.
Witt, W., Baldus, B., Bringmann, P., Cashion, L., Donner, P. and Schleuning, W.D.: Thrombolytic properties of Desmodus rotundus (vampire bat) salivary plasminogen activator in experimental pulmonary embolism in rats. Blood 79:1213-1217, 1992.
Yonenaga, Y., Frota-Fessoa, O. and Lewis, K.R.: Karyotypes of seven species of Brazilian bats. Caryologia 22:63, 1969.