| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: March 26, 2007. |
Kobus kob thomasi
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
The Uganda kob is a grazer and a close relative of the waterbucks; it is still fairly abundant in western Uganda but has become rare or extinct in Kenya and Tanzania . Two possible subspecies with somewhat different phenotypes and mtDNA findings exist (formerly ‘ Adenota' : Kobus kob; Kobus vardoni [or puku] see Nowak, 1999). Adult males weigh around 95 kg, females are 60 kg. Males are generally larger and darker than females and possess lyre-shaped horns. Kobs live gregariously in ‘leks' or ‘arenas'. The longevity of various kob species has been summarized by Weigl (2005); that of Kobus kob is given as 18-21 years. ‘Kobus' is said to be an African native name (Gotch, 1979).
The evolution of the Neotraginae is poorly understood because of the paucity of African fossils (Thenius & Hofer, 1960).
 |
Male Uganda kob at San Diego Zoo. |
2) General Gestational Data
Gestation is from 235 to 240 days and produces single neonates that weigh around 5 kg (Buechner et al., 1966). Longer gestations are quoted by Mentis (1972).
3) Implantation
Hradecky et al. (1988) described the early attachment (dorsal curvature – close to the mesometrium) of ruminant cotyledons and their future development as well as the histiotrophic nutrition of the fetus. They also depicted the implanted cotyledons of an Uganda kob. Of the various species studied by these authors they considered the kob to have the ‘simplest' placentome structure. Implantation is always in the right horn (Buechner, 1961; Mossman & Duke, 1973), as this is always the larger uterine horn.
4) General Characterization of the Placenta
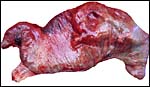
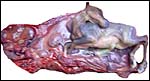
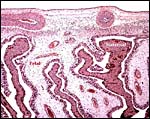
This is a polycotyledonary, epitheliochorial placenta and the only specimen available came from a pregnant animal that was euthanized because of severe trauma. The female fetus weighed 1,550 g, had a 33 cm CR length and was located in the right uterine horn. There were approximately 35 cotyledons in this gestation and the cotyledons had the ‘flat' appearance, as suggested by Mossman (1987). The corpus luteum was also on the right but the placenta extended into the left horn with smaller cotyledons. The cotyledons were arranged in four rows and the largest measured 5x4 cm. The entire placenta measured 50x20 cm and weighed 1,250 g with the uterus attached.
5) Details of fetal/maternal barrier
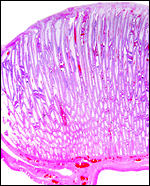
This is a typical epitheliochorial relationship with the slender and non-branching villi interdigitating with the endometrium of maternal caruncles. Binucleate cells are abundant but there was no ‘hemophagous zone'.
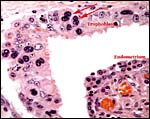 |
Trophoblast with binucleate cells (arrows) on the fetal surface with indenting maternal endometrium. |
6) Umbilical cord
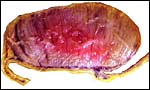
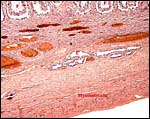
The umbilical cord of this immature gestation was 13 cm long, had four vessels and an allantoic duct as well as diminutive callosities (squamous metaplasia). The cord had no twists but contained numerous smaller blood vessels, some of which surrounded the allantoic duct.
 |
Squamous metaplasia on the surface of the umbilical cord. |
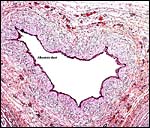 |
Centrally positioned allantoic duct in the umbilical cord. |
7) Uteroplacental circulation
This has not been described in detail.
8) Extraplacental membranes
Hradecky et al. (1988) depicted the allantoic vascularization in kobs (their Fig. 4) where ‘milky patches' were produced in the early membranes from caruncular absorption. The allantoic sac was ‘sausage-shaped' and was filled with urine.
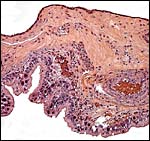 |
Membranes between cotyledons. The trophoblast (bottom) is positioned over the endometrium. |
9) Trophoblast external to barrier
There is no ‘invasion' of the uterus by trophoblast.
10) Endometrium
There is no decidualization but the endometrium has a ‘compact zone'.
11) Various features
Mossman & Duke (1973) described ‘two classes of luteal cells' in the corpora lutea of kob and some other species. They were skeptical of these being different developmental stages as described by Morrison (1971). Moreover, while this (and some other) species have smaller left uterine horns, their ovaries are bilaterally symmetrical.
12) Endocrinology
No endocrine studies are known to me.
13) Genetics
The Uganda kob has 50 chromosomes, as first identified by Taylor et al. (1967). Defassa and Ellipsen waterbucks have higher numbers as described by Kingswood et al (1998). Later, these authors described the extensive Robertsonian fusion system occurring in the kob species (Kingswood et al., 2000). Matthee & Davis (2001) studied nuclear DNA of Bovidae and concluded that the ‘problematic Reduncinae' appeared to be earliest diverging of the Caprinae/Alcelaphinae/Hippotraginae clade. Mitochondrial DNA studies by Birungi & Arctander (2000) disclosed significant differences in the mtDNA cytochrome b gene control region between the two presumed subspecies' colonies. The most comprehensive review of phylogenetic relationships in the Ruminantia comes from Hernandez Fernandez & Vrba (2005). Hybridization has taken place with Defassa waterbuck ( K. defassa ) and the Nile Lechwe ( K. megaceros ) according to Gray (1972).
 |
Male and female chromosome sets from Hsu & Benirschke (1969). |
 |
Giemsa-banded male karyotype from CRES. |
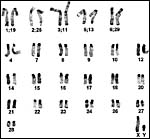 |
C-banded male karyotypes from Kingswood et al. (2000). |
14) Immunology
I am not aware of any type of immunological study in this species.
15) Pathological features
Baumeister et al. (1983) identified rotaviruses in the stools of waterbucks and many other zoo ruminants. A new species of Onchocerca (O. hamoni) was described to occur by Denke & Bain (1981). Trauma and perinatal deaths were the commonest entries in Griner's large experience (1983). Occasional other maladies cited were splenitis, malnutrition, cataracts, brachygnathia, uterine ‘adenoma' and pulmonary hemangiomas. Infectious causes of death were not mentioned.
16) Physiologic data
Hagey et al. (1997) have described the composition of bile acids in Kobus and other Artiodactyla. Pospisil et al. (1984) have described hematological values of several kob species and other artiodactyla. Kupper et al. (1981) reported on medicines used for immobilization of these animals that these are quickly reversed with nalorphine, although this was less successful than in hartebeest. Data on the composition of the ‘fetal fluids' of two waterbuck species may be found in Hradecky (1984).
17) Other resources
A wide variety of fibrous tissue cell strains from the Reduncinae is available from CRES by contacting Dr. O. Ryder (oryder@ucsd.edu).
18) Other remarks – What additional Information is needed?
The study of early stages of implantation is highly desirable.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society of San Diego. I am also grateful to the pathologists at the San Diego Zoo for allowing me to obtain this specimen.
References
Baumeister, B.M., Castro, A.E., McGuire-Rodgers, S.J. and Ramsay, E.C.: Detection and control of rotavirus infections in zoo animals. J. Am. Vet. Med. Assoc. 183:1252-1254, 1983.
Birungi, J. and Arctander, P.: Large sequence divergence of mitochondrial DNA genotypes of the control region within populations of the African antelope, genus kob (Kobus kob). Mol. Ecol. 9:1997-2008, 2000.
Buechner, H.K.: Unilateral implantation in the Uganda kob, Adenota kob thomasi (P.S. Sclater). Nature 190:738, 1961.
Buechner, H., K., Morrison, J.A. and Leuthold, W.: Reproduction in Uganda kob with special reference to behaviour. Symp. Zool. Soc. London 15:69-88, 1966.
Denke, A.M. and Bain, O.: Two new nodular Onchocerca spp. in wild Bovidae in Upper Volta . Ann. Parasitol. Hum. Comp. 56:339-347, 1981.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole , Dorset, 1979.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2 nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A. : Pathology of Zoo Animals. Zoological Society of San Diego , San Diego , California , 1983.
Hagey, L.R., Gavrilkina, M.A. and Hofmann, A.F.: Age-related changes in the biliary bile acid composition of bovids. Canad. J. Zool. 75:1193-1201, 1997.
Hernandez Fernandez, M. and Vrba, E.S.: A complete estimate of the phylogenetic relationships in Ruminantia: a dated species-level supertree of the extant ruminants. Biol. Rev. Camb. Philos. Soc. 80:269-302, 2005.
Hradecky, P.: Composition of the fetal fluids in African antelopes. Theriogenology 22:657-665, 1984.
Hradecky, P., Mossman, H.W. and Stott, G.G.: Comparative development of ruminant placentomes. Theriogenology 29:693-729 and 715-729, 1988.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag , New York Vol. 3, Folio 143, 1969.
Kingswood , S.C. , Kumamoto , A.T., Charter, S.J., Aman, R.A. and Ryder, O.A.: Centric fusion polymorphism in waterbuck (Kobus ellipsiprymnus). J. Hered. 89:96-`00, 1998.
Kingswood , S.C. , Kumamoto , A.T., Charter, S.J., Houck, M.L. and Benirschke, K.: Chromosomes of the antelope genus Kobus (Artiodactyla, Bovidae): karyotypic divergence by centric fusion rearrangements. Cytogenet. Cell Genet. 91:128-133, 2000.
Kupper, W., Drager, N., Mehlitz, D. and Zillmann, U.: On the immobilization of hartebeest and kob in Upper Volta . Tropenmed. Parasitol. 32:58-60, 1981.
Matthee , C.A. and Davis , S.K.: Molecular insights into the evolution of the family Bovidae: A nuclear DNA perspective. Mol. Biol. Evol. 18:1220-1230, 2001.
Mentis, M.T.: A review of some life history features of the large herbivores of Africa . The Lammergeyer16:1-89, 1972.
Morrison, J.A.: Morphology of corpora lutea in the Uganda kob antelope, Adenota kob thomasi (Neumann). J. Reprod. Fertil. 26:297-305, 1071.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison , Wisconsin , 1973.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Pospisil, J., Kase, F., Vahala, J. and Mouchova, I. : Basic haematologicl values in antelopes – III. The reduncinae and the antilopinae. Comp. Biochem. Physiol. A 78:809-813, 1984.
Taylor , K.M., Hungerford, D.A. and Snyder, R.L.: The chromosomes of four artiodactyls and one perissodactyl. Mamm. Chromos. Newsl. 8:233, 1967.
Thenius, E. and Hofer, H.: Stammesgeschichte der Säugetiere. Springer-Verlag, Berlin, 1960.
Weigl, R.: Longevity of Mammals in Captivity; From the Living Collections of the World. [Kleine Senckenberg-Reihe 48]. E. Schweizerbart'sche Verlagsbuchhandlung (Nägele und Obermiller), Stuttgart, 2005.