| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
Tupaia glis
by Peter Kaufmann
Order: Primates (Scandentia)
Family: Tupaiidae
1) General Zoological Data
There are 11 species in this family according to Nowak (1999), with this species the perhaps being commonest tree shrew. They are easily kept in captivity and are occasionally seen in zoos. "Tupai" is the Malay world for squirrels because these animals resemble squirrels closely. Tree shrews are diurnal animals with strong pair bonding; they nest in trees and shrubs. Males are dominant and mate with several females. Because of their external features and other structural similarities, tupaias have repeatedly been classified as a branch of insectivores. Comparative anatomical (Le Gros Clarc, 1962) and serological features (Moore & Goodman, 1968), however, have indicated that the tree shrews are lower primates which, phylogenetically, originate from the basal stock of primate evolution. Similarities in tarsal morphology suggest that tupaias, together with primates and bats, originated from the 'archontan stock' of placental mammals which first developed the capability of living in trees (Szalay & Drawhorn, 1980; Adkins & Honeycott, 1991). Their affiliation to primates and to the archontan stock was further supported by sequence analysis of several proteins (Cronin & Sarich, 1980).
Tupaias have aroused increasing interest as laboratory animals because of
their taxonomic affiliation to primates, their endocrine control of pregnancy which shows striking similarities to that of humans (see below), their small body weight (<200g), and their rather long life span (longevity record of nine years, according to Kuhn & Schwaier, 1973; but 12+ years according to Jones (1980).
 |
Adult tree shrew (Tupaia glis). |
Length of gestation: 41 - 45 days
Litter size: 1 to 4, mean 2
Body weight (non pregnant) 190 g, at full term 240 g
Fetal weight at full term: 11 - 15 g
Weight of placental discs and membranes at full term: 0.5 - 0.6 g
The neonatal/placental weight ratio is 20:1 to 30:1 (Dantzer et al., 1988) and thus makes this one of the most effective placentas ever studied. For comparison, the respective ratio of the human gestation is about 6:1. It is surprising that so little placental weight can supply so much fetal weight, in particular since the placenta looks microscopically far less effective than those of e.g. caviomorph rodents that have the same or an even slightly lower weight ratio. When compared to the guinea pig placenta, the tupaia placenta contains much more connective tissue to the debit of trophoblast (Kaufmann et al., 1985). It has an endotheliochorial barrier with a mean diffusion length clearly longer than that of the guinea pig (Kaufmann et al., 1985), has a cross-current flow arrangement of vessels (Luckhardt et al., 1985; Dantzer et al., 1988) which is considered to be less effective than the counter-current flow of the guinea pig placenta (Dantzer et al., 1988).
3)
Implantation
Tupaias have a superficial, mesometrial implantation around day 6 post
conception (Kuhn & Schwaier, 1973).
4) General Characterization of the Placenta
General placental type: Bidiscoidal, labyrinthine, endotheliochorial,
chorioallantoic placenta with choriovitelline membranes (Luckett,1968;
Kuhn & Schwaier, 1973; Schwaier et al., 1976; Luckhardt et al., 1985).
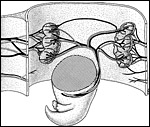
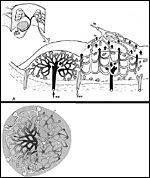
Gross anatomy: The Malayan tree shrew possesses two uterine
horns, each horn providing space for one to three implantation sites.
The elongated uterine horns unite in a short common corpus. Each fetus
is connected to two cushion-like placentas that are situated at the anterior
and posterior walls of the uterus (see the following diagram). Each placental
disc consists of three large lobes and several attached smaller lobes
that together form a kidney-shaped placental disc (second diagram).
Allantois: The allantois vascularizes the chorion to
form the two chorioallantoic placental discs, and it contacts the antimesometrial
and lateral regions of the amnion to form an amnio-allantoic membrane.
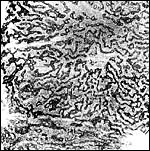
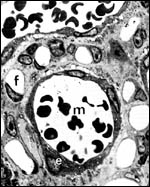
The disc-like lobes are composed of a meshwork of slender trophoblast trabeculae, which contain maternal capillaries lined by maternal endothelium (next diagram). The trophoblast trabeculae are separated by ample connective tissue into which fetal capillaries are embedded. Consequently, the materno/fetal barrier belongs to the endothelio-chorial type. It consists of the following layers: Maternal endothelium lining the maternal blood space; the endothelial basal lamina; a multinucleated trophoblast layer, which structurally resembles a syncytiotrophoblast; the complete absence of cytotrophoblast throughout the second half of gestation, the unusually large, euchromatic nuclei and the presence of centrioles raise, however, the question whether this is really a true syncytium, generated and growing by cell-fusion; as alternative it may be considered a plasmodium, growing by nuclear division without subsequent cytoplasmic division - this cannot be ruled out completely; the trophoblast basal lamina; fetal connective tissue cells of different types; fetal endothelium, lining the fetal capillaries, without their own basal lamina (Luckhardt et al., 1985; Kaufmann et al., 1985).
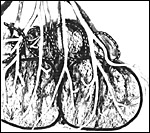
The rather long, slightly twisted umbilical cord surrounds the fetal abdomen, branches on its back into two bundles of arteries and veins which supply both placental discs (Figure 2 and below). Numerous fetal arterioles and venules bend vertically into the labyrinth from the surface of the lobules of both placental discs. There they branch into networks of numerous radially arranged fetal capillaries with average lengths of 0.5 to 1mm.
The maternal vascular supply is that of all mammals with a bicornuate uterus: Ovarian arteries and veins, derived from the aorta or from the renal vessels, and uterine arteries branch off the inner iliac artery, unite and form two long arcade arteries and veins located medial to the uterine horns. From these arcade vessels 5 to 10 mesometrial/radial arteries and veins branch off which supply the implantation sites via uteroplacental vessels as well as uterine segments without implantation sites via myometrial vessels (previous diagram). When leaving the mesometrium and entering the uterine horns, the uteroplacental arteries branch into anterior branches for the anterior placental disc and posterior branches for the respective posterior disc.
Upon reaching the placental discs, one uteroplacental arteriole per lobule penetrates into the center of the lobule where it branches into a network of capillaries (next diagram). The latter are collected by several veins which are arranged around the lobules. Accordingly, each lobule represents one maternal circulatory unit with rather long maternal capillaries, averaging 3 to 4 mm in length (previous diagram at upper left). In each of these large maternal circulatory units, 10 to 15 fetal circulatory units with correspondingly shorter capillaries are integrated (previous diagram at lower left). Therefore, maternal and fetal capillaries cross each other in varying angles. The resulting flow conditions can be described best as a crosscurrent flow exchanger system (Dantzer et al., 1988).
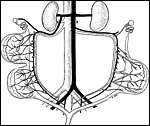 |
Maternal arterial (black) and venous (white) supplies of uterine horns and placental discs. |
The thinner interplacental parts of the fetal membrane sac become reduced to a rudimentary layer of trophoblast cells which face the endometrium (corresponding to the chorion laeve in the human placenta) during early stages of pregnancy. The fetal surface of this rudimentary trophoblast layer is covered by two layers of vascularized yolk sac wall, followed by an innermost layer of amnion (Kuhn & Schwaier, 1973).
9) Trophoblast External to Barrier
To the best of our knowledge, nearly all studies of the tupaia placenta
have focused on the exchange areas. Whether trophoblast cells leave the
placental discs and interstitially invade the maternal host tissue is
thus presently unknown. Also, the question of an endovascular invasion
of uteroplacental vessels with respective pregnancy adaptation of arteries
remains presently unsolved.
10) Endometrium
Close to the placentas, endometrial stromal cells show decidualization.
Locally, so-called decidual knots, multinucleated decidual cells, can
be observed.
11) Various Features
Maeda et al. (1996) studied the seminiferous epithelium is Tupaia glis
and suggested that spermatogenesis differs from that of higher primates
and also from rodents.
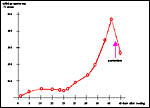
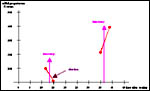
Hormonal control of pregnancy shows striking similarities between tupaias and humans (Schwaier et al., 1976; Elger & Hasan, 1984, unpublished): The course of serum progesterone levels in early (days 12 to 15 p.c.) and late pregnancy (days 35 to 40 p.c.) suggests a luteo-placental shift of progesterone secretion around day 20 post conception.
Parturition occurs in the presence of high serum progesterone levels, which cease only after parturition.
Kimura et al. (2000) localized several enzymes (aromatase, etc.) and inhibin to the ovaries.
13) Genetics
Tupaia glis has 60 chromosomes as shown below (Hsu & Benirschke, 1968), but other authors have cited there have found 2n=62; Tupaia chinensis has 2n=62, and Tupaia montana has 2n=68 chromosomes. Perhaps species designation has been uncertain in some specimens studied, although sympatric tree shrew populations with 60 and 62 chromosomes have been identified in Thailand (Hirai et al., 2002). Hybrid cytotypes were not observed. Their 2n=62 cytotype was assigned to T. belangeri. More recent sudies have suggested Robertsonian fusion mechanisms as being responsible for the reduction from 2n=62 to 2n=60 (Toder et al., 1992). Toder et al. (1993) later also described chiasmatic pairing between X- and Y-chromosomes.
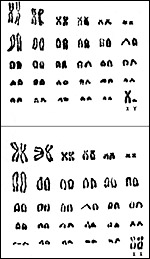 |
Karyotypes of Tupaia glis with 2n=60. |
I am not aware of any relevant data.
15) Pathological Features
Griner (1983) reported myocardial necrosis and depicted severe scoliosis
in tree shrews. The scoliotic animal also had ankylosing arthritis. A
number of parasites have been identified in three shrews, the literature
to which is accessible through PubMed.
16) Physiological Data
According to the vascular arrangement described above, the maternal/fetal
flow conditions can be described best as a cross-current flow exchanger
system. Tree shrews have been investigated anatomically especially for
their neural anatomy. Relevant literature is accessible through PubMed.
17) Other Resources
At CRES
(San Diego Zoo) fibroblast cell strains are available of only two specie
of tree shrews: Tupaia minor and Tupaia tana. They can
be made available by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other Remarks - What additional
information is needed?
There is imperfect knowledge of the depth of trophoblastic invasion into
blood vessels.
Acknowledgement
The tupaias were derived from a colony of the Schering Company, Berlin,
Germany. Dr. Walter Elger, Schering Company kindly provided the placentas
as well as most of the endocrine data.
References
Adkins, R.M. and Honeycutt, R.L.: Molecular phylogeny of the superorder
Archonta. Proc. Natl. Acad. Sci. USA 88:10317-10321, 1991.
Clark Le Gros W.E.: The Antecedents of Man. University Press, Edinburgh, 2nd ed. (1962).
Cronin, J.E. and Sarich, V.M.: Tupaiid and archonta phylogeny: the macromolecular evidence. In, Luckett, W.P. (ed.) Comparative Biology and Evolutionary Relationships of Tree Shrews. Plenum Press, New York-London, 1980.
Dantzer, V., Leiser, R., Kaufmann, P. and Luckhardt, M.: Comparative aspects of placental vascularization. Trophoblast Research 3:235-260, 1988.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hirai, H., Hirai, Y., Kawamoto, Y., Endo, H. Kimura, J. and Rerkamnuaychoke, W.: Cytogenetic differentiation of two sympatric tree shrew taxa found in the southern part of the Isthmus of Kra. Chromosome Research 10:313-327, 2002.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Springer-Verlag NY. Volume 2, Folio 97, 1968.
Jones, M.L.: Lifespan in mammals. In, The Comparative Pathology of Zoo Animals. R.J. Montali and G. Migaki, eds. Pp. 495-515. Smithsonian Institution Press, Washington D.C. 1980.
Kaufmann, P., Luckhardt, M. and Elger, W.: The structure of the tupaia placenta. II. Ultrastructure. Anat. Embryol. 171:211-221, 1985.
Kimura, J., Tsukise, A., Watanabe, G., Taya, K., Rerkamnuaychoke, W., Endo, H., Kurohmaru, M., Yamada, J. and Nishida, T.: Immunohistochemical localization of inhibin and steroidogenic enzymes in the ovary of common tree shrew (Tupaia glis) and northern smooth-tailed tree shrew (Dendrogale murina). Anat. Histol. Embryol. 29:267-271, 2000.
Kuhn, H.J. and Schwaiger, A.: Implantation, early placentation and the chronology of embryogenesis in Tupaia belangeri. Anat. Embryol. 142:315-340, 1973.
Luckett, W.P.: Morphogenesis of the placenta and fetal membranes of the tree shrews. Amer. J. Anat. 123:385-428, 1968.
Luckhardt, M., Kaufmann, P. and Elger, W.: The structure of the tupaia placenta. I. Histology and vascularisation. Anat. Embryol. 171:201-210, 1985.
Maeda, S., Endo, H., Kimura, J., Rerkamnuaychoke, W., Chungsamarnyart, N., Yamada, J., Kurohmarum, Hayashi, Y. and Nishida, T.: Classification of the cycle of the seminiferous epithelium in the common tree shrew (Tupaia glis). J. Vet. Med.. Sci. 58:481-414, 1996.
Moore, W.G. and Goodman, M.: A set theoretical approach to immunotaxonomy. Analysis of species comparison in modified Ouchterlony plates. Bull. Math. Biophys. 30:289-289, 1965.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Schwaier, A. , Kuhn, H.J. and Hasan, H.: Serum progesterone levels during pregnancy in Tupaia belangeri correlated with ovarian and placental morphology. In, Antikatzides T.S. Erichsen & A. Spiegel (eds.). The Laboratory Animal in the Study of Reproduction. Fischer Publishers, Stuttgart-New York, 1976.
Szalay, F.S. and Drawhorn, G.: Evolution and diversification of archonta in an arboreal milieu. In, Luckett, W.P. (ed.) Comparative Biology and Evolutionary Relationships of Tree Shrews. Plenum Press, New York-London, 1980.
Toder, R., von Holst, D. and Schempp, W.: Comparative cytogenetic studies in tree shrews (Tupaia). Cytogenet. Cell Genet. 60:55-59, 1992.
Toder, R., Rumpler, Y., von Holst, D. and Schempp, W.: An X-Y homologous pairing segment in tree shrews (Tupaia). Cytogenet. Cell Genet. 63:135-140, 1993.