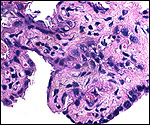
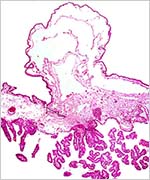
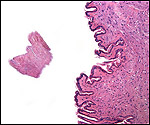
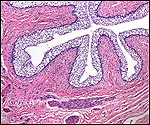
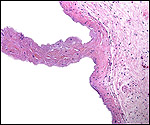
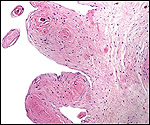
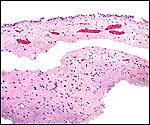
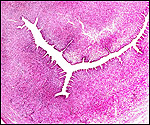
| Last updated: July 19, 2010. |
Tapirus indicus
Order: Perissodactyla
Family: Tapiridae
1) General Zoological Data
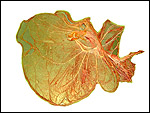
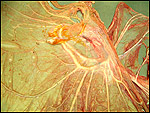
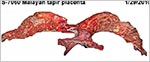
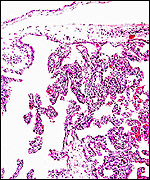
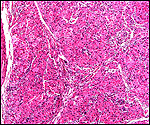
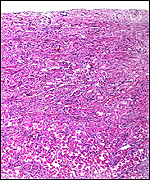
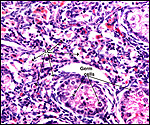
Of the four species of tapirs, Tapirus indicus is the only Asiatic form; the other three species are South American tapirs. The Malayan tapir is characterized by its white saddle and larger size. The saddle is also the reason for its name "saddle backed tapir" or "Schabrackentapir", in German. The young of all species have fine white stripes on brown skin. Malayan tapirs are generally solitary animals. They are the largest of the four species, weighing in excess of 350 kg. The females are usually somewhat larger than males. Tapirs need warm environments in order to thrive in captivity, and they must ideally have access to a pool. Tapirs are excellent swimmers. The longevity of this species is over 30 years in captivity. Many animals are kept and bred in various zoological gardens. All tapirs are endangered species.
The name "tapir" derives from a South American Indian tribe, the Tupi (Gotch, 1979). The designation "indicus", however, is misleading, as these animals do not come from India. Rather, they are "East Indian" from Sumatra and the Malayan peninsula. Some taxonomists have preferred to assign the genus name "Acrocodia" to the Malayan tapir because of its being somewhat distinct from the South American forms. There is a detailed description of morphology and phylogeny in Starck (1995). Barongi (1993) presented an inclusive report on the management and conservation of tapirs in 1993.
The tapiridae were once distributed world-wide and the East Indian and American tapirs are believed to have diverged from one another about 20-30 MYA (Ashley et al., 1996). The South American tapirs immigrated about 3 MYA from North America where they became extinct. Between 1000-3000 Malayan tapirs may still be in the wild, according to Ashley et al. (1996).