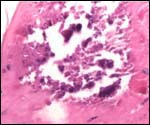
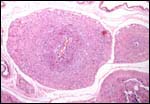
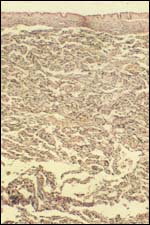
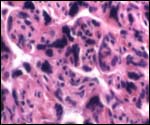
| |
16) Physiological data
No relevant data are reported
17)
Other resources
A number of fibroblast cell lines are available through CRES, the research
arm of the Zoological Society of San Diego (see: http://www.sandiegozoo.org/conservation/cres_home.html.
Please direct your inquiries to Dr. Oliver Ryder (oryder@ucsd.edu).
18)
Additional needs for study
We know too little about the frequency of demise of one of the twins.
In human gestation, this can be due to the so-called twin-to-twin-transfusion
syndrome, or it may be due to the conversion into acardiac fetus because
of the presence of vascular anastomoses. How often does this happen in
marmosets and tamarins? Also, the real physiologic reason why the female
twin, who is chimeric with the male co-twin, is spared the freemartin
syndrome of artiodactyls (cattle especially) is not understood.
References
There is a very extensive bibliography on golden lion tamarins. An especially
important reference is:
Kleiman, D.: Social and sexual behaviour of Leontopithecus rosalia
during the reproductive cycle. In, Kleiman, D., The Biology and Conservation
of the Callithricidae . Smithsonian Press, Washington, 1976.
Barnhart,
D.D. and Gengozian, N.: An evaluation of the mixed lymphocyte culture
reaction in marmosets. Transplantation 20:107-115, 1975.
Bedard,
M.T., Ma, S.F. and Jones, T.C.: Chromosomal banding patterns and nucleolar
organizing regions in three species of Callithricidae (Saguinus oedipus,
Saguinus fuscicollis, and Callithrix jacchus). J. Med. primatol.
7:82-97, 1978.
Bender,
M.A. and Mettler, L.E.: Chromosome studies of primates. II. Callithrix,
Leontocebus and Callimico. Cytologia (Tokyo) 25:400-404,
1960.
Benirschke,
K., Anderson, J.M. and Brownhill, L.E.: Marrow chimerism in marmosets.
Science 138:513-515, 1962.
Benirschke,
K. and Brownhill, L.E.: Further observations on marrow chimerism in marmosets.
Cytogenetics 2:331-341, 1963.
Benirschke, K. and Layton, W.: An early twin blastocyst of the golden
lion marmoset, Leontocebus rosalia L. Fol. Primatol. 10:131-138,
1969.
Cadavid,
L.F., Shufflebotham, C., Ruiz, F.J., Yeager, M., Hughes, A.L. and Watkins,
D.I.: Evolutionary instability of the major histocompatibility complex
class I loci in New World primates. Proc. Natl. Acad. Sci. USA 94:14536-14541,
1997.
Cell
lines inquiries: Dr. Oliver Ryder (oryder@ucsd.edu).
Chalifoux,
L.V. and Elliott, M.W.:Congenital anomalies in two neonatal tamarins (Saguinus
oedipus and Saguinus fuscicollis). J. Med. Primatol. 15:329-337,
1986.
Chambers,
P.L. and Hearn, J.P.: Embryonic, foetal and placental development in the
common marmoset monkey (Callithrix jacchus). J. Zool. London 207:545-561,
1985.
Coimbra-Filho,
A.F. and Mittermeier, R.A.: New data on the taxonomy of Brazilian marmosets
of the genus Callithrix Erxleben, 1777. Folia primatol. 20:241-264, 1973.
Coimbra-Filho,
A.F. and Mittermeier, R.A., eds.: Ecology and Behavior of Neotropical
Primates. Academia Brasileira de Ciências. Rio de Janeiro, 1981.
Cooper,
R.W.: Small species of primates in biomedical research. Lab. Anim. Care
18:267-279, 1968.
Cronin,
J.E. and Sarich, V.M.: Molecular systematics of the New World monkeys.
J. Human Evol. 4:357-375, 1975.
Dunn,
F.L.: Acanthocephalans and cestodes os South American monkeys and marmosets.
J. Parasitol. 49:717-722, 1963.
Enders, A.C. and Lopata, A.: Implantation in the marmoset monkey: Expansion
of the early implantation site. Anat. Rec. 256279-299, 1999.
Flurer,
C.I. and Zucker, H.: Evaluation of serum parameters relevant to vitamin
D status in tamarins. J. Med. Primatol. 16:175-184, 1987.
Egozcue,
J., Perkins, E.M. and Hagemenas, F.: Chromosomal evolution in marmosets,
tamarins, and pinchés. Folia Primatol. 9:81-94, 1968.
Gengozian, N.: Marmosets: their potential in experimental medicine. Ann.
N.Y. Acad. Sci. 162:336-362, 1969.
Gengozian,
N. and Merritt, C.B.: Effect of unilateral ovariectomy on twinning frequency
in the marmoset. J. Reprod. Fert. 23:509-512, 1970.
Gengozian,
N., Batson, J.S. and Nelson, B.M.: Bone-marrow grafting attempts in marmosets
after whole-body irradiation. Int. J. Rad. Biol. 11:553-561, 1966.
Gengozian,
N., Lushbaugh, C.C., Humason, G.L. and Kniseley, R.M.: "Erythroblastosis
foetalis" in the primate, Tamarinus nigricollis. Nature 209:731-732,
1966.
Gray,
A.P.: Mammalian Hybrids. Commonwealth Agricultural Bureaux, Farnham Royal,
Slough SL2 3BN, 1971.
Hearn,
J.P., Abbott, D.H., Chambers, P.C., Hodges, J.K. and Lunn, S.F.: Use of
the common marmoset, Callithrix jacchus, in reproductive research.
Prim. Med. 10:40-49, 1978.
Hershkovitz,
P.: Notes on tertiary platyrrhine monkeys and description of a new genus
from the late miocene of Colombia. Folia Primatol. 12:1-37, 1970.
Hershkovitz,
P.: Comments on the taxonomy of Brazilian marmosets (Callithrix,
Callitrichidae). Folia Primatol. 24:137-172, 1975.
Hampton,
J.K., Hampton, S.H. and Levy, B.M.: Reproductive physiology and pregnancy
in marmosets. pp. 527-535 in Medical Primatology, Karger, Basel 1970.
Hearn,
J.P., Abbott, D.H., Chambers, P.C., Hodges, J.K. and Lunn, S.F.: Use of
the common marmoset, Callithrix jacchus, in reproductive research.
Prim. Med. 10:40-49, 1978. Karger, Basel.
Hobson,
B.M., Hearn, J.P., Lunn, S.F. and Flockhart, J.H.: Urinary excretion of
biologically active chorionic gonadotrophin by the pregnant marmoset (Callithrix
jacchus jacchus). Fol. Primatol. 28:251-258, 1977.
Gengozian, N.: Marmosets: their potential in experimental medicine. Ann.
N.Y. Acad. Sci. 162:336-362, 1969.
Gengozian,
N. and Merritt, C.B.: Effect of unilateral ovariectomy on twinning frequency
in the marmoset. J. Reprod. Fert. 23:509-512, 1970.
Gengozian,
N., Batson, J.S. and Nelson, B.M.: Bone-marrow grafting attempts in marmosets
after whole-body irradiation. Int. J. Rad. Biol. 11:553-561, 1966.
Gengozian,
N., Lushbaugh, C.C., Humason, G.L. and Kniseley, R.M.: "Erythroblastosis
foetalis" in the primate, Tamarinus nigricollis. Nature 209:731-732,
1966.
Gray,
A.P.: Mammalian Hybrids. Commonwealth Agricultural Bureaux, Farnham Royal,
Slough SL2 3BN, 1971.
Hearn,
J.P., Abbott, D.H., Chambers, P.C., Hodges, J.K. and Lunn, S.F.: Use of
the common marmoset, Callithrix jacchus, in reproductive research. Prim.
Med. 10:40-49, 1978.
Hershkovitz,
P.: Notes on tertiary platyrrhine monkeys and description of a new genus
from the late miocene of Colombia. Folia Primatol. 12:1-37, 1970.
Hershkovitz,
P.: Comments on the taxonomy of Brazilian marmosets (Callithrix, Callitrichidae).
Folia Primatol. 24:137-172, 1975.
Hampton,
J.K., Hampton, S.H. and Levy, B.M.: Reproductive physiology and pregnancy
in marmosets. pp. 527-535 in Medical Primatology, Karger, Basel 1970.
Hearn,
J.P., Abbott, D.H., Chambers, P.C., Hodges, J.K. and Lunn, S.F.: Use of
the common marmoset, Callithrix jacchus, in reproductive research. Prim.
Med. 10:40-49, 1978. Karger, Basel.
Hobson,
B.M., Hearn, J.P., Lunn, S.F. and Flockhart, J.H.: Urinary excretion of
biologically active chorionic gonadotrophin by the pregnant marmoset (Callithrix
jacchus jacchus). Fol. Primatol. 28:251-258, 1977.
Hobson, B.M. and Wide, L.: The similarity of chorionic gonadotrophin and
its subunits in term placentae from man, apes, old and new world monkeys
and a prosimian. Fol. Primatol. 35:51-64, 1981.
Hunt,
R.D. and Garcia, F.G.: Hypervitaminosis D in New World monkeys. Am. J.
Clin. Nutrition 22:358-366, 1969.
86:221-223, 1963.
Hunt,
R.D., Garcia, F.G. and Hegsted, D.M.: A comparison of vitamin D2 and D3
in New World primates. I. Production and regression of osteodystrophia
fibrosa. Lab. Anim. Care 17:222-234, 1967.
Jollie,
W.P., Haar, J.L. and Craig, S.S.: Fine structural observations on hematopoiesis
in the chorioallantoic placenta of the marmoset. Amer. J. Anat. 144:9-38,
1975.
Lasley,
B.L., Monfort, S.L., Hodges, J.K. and Czekala, N.M.: Comparison of urinary
estrogens during pregnancy in diverse species. In, Fetal Endocrinology,
pp. 111-126, Academic Press, 1981.
Merker, H.-J., Bremer, D., Barrach, H.-J. and Gossrau, R.: The basement
membrane of the persisting maternal blood vessels in the placenta of Callithrix
jacchus. Anat. Embryol. 176:87-97, 1987.
Nowak,
R.M. and Paradiso, J.L.: Walker's Mammals of the World, 4th ed. Vol. 1.Johns
Hopkins Univ. Press, Baltimore, 1983.
Pauly, A. and Strauss, G.: Kaiserschnit bei einem Goldkopflowenaffchen
(Leontopithecus chrysomelas) mit Entwicklung einer mummifizierten
Frucht. Milu 10:583-587, 2002.
Preslock,
J.P., Hampton, S.H. and Hampton, J.K.: Cyclic variations of serum progestins
and immunoreactive estrogens in marmosets. Endocrinology 92:1096-1101,
1973.
Ryan,
K.J., Benirschke, K. and Smith, O.W.: conversion of andro-stenedione-4-C14
to estrone by the marmoset placenta. Endocrinology 69:613-618, 1961
Sarich,
V.M. and Cronin, J.E.: South American mamma molecular systematics, evolutionary
clocks, and continental drift. In, Evolutionary Biology of the New World
Monkeys and Continental Drift. R. Ciochon & A.B. Chiarelli, eds. Plenum
Press, N.Y. 1980. Chapter 20.
Seuanez,
H.N., L. Forman, L. and Alves, G.: Comparative chromosome morphology in
three Callithrichid genera: Cebuella, Callithrix, and Leontopithecus.
J. Hered. 79:418-424, 1988.
Smith,
C.A., Moore, H.D.M. and Hearn, J.P.: The ultrastructure of early implantation
in the marmoset monkey (Callithrix jacchus). 175:399-410, 1987.
Spatz,
W.B.: Nabelschnur-Länge bei Insektivoren und Primaten. Z. Säugetierk.
33:226-239, 1968.
Turton,
J.A., Ford, D.J., Bleby, J., Hall, B.M. and Whiting, R.: Composition of
the milk of the common marmoset (Callithrix jacchus) and milk substitutes
used in hand-rearing programmes, with special reference to fatty acids.
Fol. Primatol. 29:64-79, 1978.
Windle,
C.P., Baker, H.F., Ridley, R.M., Oerke, A-K. and Martin, R.D.: Unrearable
litters and prenatal reduction of litter size in the common marmoset (Callithrix
jacchus). J. Med. Primatol. 28:73-83, 1999.
Wislocki,
G.B.: Hematopoiesis in the chorionic villi of the placenta of platyrrhine
monkeys. Anat. Rec. 85:349-363, 1932.
Wislocki, G.B.: Observations on twinning in marmosets. Amer. J. Anat.
64:445-483, 1939.
Wislocki, G.B.: Placentation in the marmoset (Oedipomidas geoffroyi)
with remarks on twinning in monkeys. Anat. Rec. 52:381-399, 1932.
Wynn, R.M., Richards, S.C. and Harris, J.A.: Electron microscopy of the
placenta and related structures of the marmoset. Amer. J. Obstet. Gynecol.
122:60-69, 1975.
Wynn,
R.M., Richards, S.C. and Harris, J.A.: Electron microscopy of the placenta
and related structures of the marmoset. Amer. J. Obstet. Gynecol. 122:60-69,
1975.
Moore, H.D.M., Gems, A. and Hearn, J.P.: Early implantation stages in
the marmoset monkey (Callithrix jacchus). Amer. J. Anat. 172:265-278,
1985.
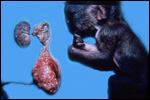
Various species of marmosets/tamarins
|