| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Dec 25, 2011 |
Sri Lankan Slow (Slender) Loris
Loris tardigradus
Order: Primates
Suborder: Strepsirhini
Infraorder: Loriformes
Family: Loridae (Lorisidae)
1.General Zoological Data
More than one species may be subsumed under this species which also has been described to have several subspecies. This nocturnal animal has very large eyes and is arboreal in its normal habitat; in fact, it is very difficult to remove an animal from the branches of trees because of its firm attachment by hands and feet. It is often insectivorous but also eats fruit, leaves, eggs, nuts, small mammals, lizards etc.; it is thus essentially omnivorous. The species (or its subspecies) comes from Southern India and mostly from Sri Lanka. These animals are distinctly slower than galagos, and from their hands and feet they leave behind urinary secretions that are species-specific.
The female fetus of this gravida 1 mother weighed 12.4 g and was covered with fine white hair. Upon opening the chest the lung floated in formalin solution. Adults weigh between 85 and 348 g according to Nowak and Paradiso, 1983.
 |
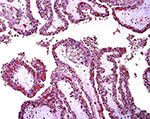
Slender loris specimen as it was received. |
2. General Gestational Data
Gestation lasts about 122 days (Kolar & Wendt, 1967) (or 193 days according to Nowak & Paradiso, 1983), but Manley (1966, 1967) describes that the gestation lasts 163 days. One or, less commonly two young result, and gestations are possible twice annually. The young remain with the mother for over one year. Life expectancy is at least 7 years but Nowak and Paradiso (1983) stated that life expectancy is between 12 and 14 years.
3. Implantation
Early implantational studies are not available for this particular species but they are badly needed. They were detailed for its relative, Nycticebus lydekkerianus (=tardigradus) by Hill et al. (1928) who observed three early embryos. It is likely that pygmy lorises have a very similar early development.
4. General Characterization of the placenta
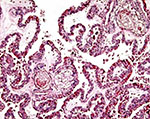
Mossman (1987) suggested that the placentas of the pygmy loris must be similar to those of other Lorisidae (such as the slender Loris and Galago). Those placentas have been observed, especially that of the galago, and they are referred to by Mossman. He described them to be typical of the Strepsirhini and they have a diffuse villous, epitheliochorial type of placenta. That is to say that they have a diffuse microvillous structure, are surrounded by a “ring of allantochorion”, have ‘areolae’, and their nidation is superficial; they also have a temporary juxtauterine omphalopleure and a large, lobed allantois. The areolae of Loris lydekkerianus (see also Hill et al., 1928, and according to Enders [personal communication, 2008], the picture shown here is of the placenta from the slender loris, L. tardigradus. This specimen was also further described on p. S13 by Enders & Carter, 2006) and they were depicted by Mossman (1987) as being located and being contiguous with the ‘uterine milk’-producing endometrial glands, although they were then shown to contain much more secretion than is the case in the specimen of pygmy loris. Moreover, since that specimen had been of a delivered placenta without attachment to the uterus, the identification of the localization of these areolae remains as being tentative. They were always round structures without openings to the intervillous space (at least in the sections made), and they were surrounded by a thin layer of smooth musculature that contained occasional small blood vessels (Enders & Carter, 2006 have shown similar features in color photographs on p. S13). A small amount of debris was found within them. The epithelial cells of the cristae were stained much darker than that of the villous trophoblast. It contained fine cytoplasmic vacuoles, but only little stainable iron was found as is described by others to occur in pig placentas. In contrast, the cytoplasm had numerous globular protein inclusions that were usually located beneath the nuclei and were adjacent to the rich capillary bed (shown below). They were depicted for the pygmy loris (see that chapter in this volume) but were not present in the photographs shown by Enders & Carter (2006); an occasional globular inclusion had a deep yellow-brown color, which contained some iron moiety. Importantly, the epithelium of the cristae had a much lesser staining reaction with pan-cytokeratin preparations than that of the villous trophoblast, while the wall of the areolae was deeply stained. Perhaps the epithelium has a different origin than trophoblast. Consider this possibility: might they not be ‘herniations’ into trophoblastic epithelium (their walls are deeply stained) into which endometrial epithelium grew. Only new material from attached placentas, especially young ones, and employing similar staining reactions will settle this point. Although they are commonly called ‘chorionic vesicles’, that may not be a proper appellation.
Similar placentas are often described in context with the placentas of pigs (e.g.: by Amoroso, 1961; Mossman, 1987; and Wooding & Flint, 1994). In their considerations, the trophoblast never fuses with endometrial epithelium and uterine ‘milk’ is produced by the endometrial glands and forwarded to “areolae” that are depicted by Amoroso and the other authors. These regions were described by Amoroso on pp. 174-175 as follows:
“The most conspicuous structural differentiations on the surface of the pig’s chorion are the areolae. Embryologists have long been aware of the fact that these structures which arise as minute circular areas opposite the mouths of the uterine glands, represent regions of the chorion which may have become specialised in connection with the absorption of the secretions elaborated by the glands and the uterine epithelium (von Baer, 1828; Turner, 1875; Grosser, 1927). Together with the uterine depressions which fit over them, they form definite organic units isolated structurally from the rest of the placenta. Two morphological types of areolae (called “rosettes” by Abromavich, 1926) are differentiated on the chorion of the 10 to 12 mm embryo, the regular areolae, the ones usually described in the literature, which are circular, opaque, and complexly folded, cup-like invaginations of the chorionic surface (Fig. 15.25b), and the irregular areolae (called pits by Heuser, 1927), translucent plaque-like structures with little tendency to invagination, and as much as fifteen times the diameter of the regular areolae (Fig. 15.25a). During very early stages of gestation, the surface of the regular areolae is flat (Fig. 15.26a), but as gestation advances the flat surface is invaginated and becomes complicated by ridges and papilla-like structures (Fig. 15.26b). The two structural types, occurring in large numbers (8000-9000), are distributed over the entire chorion and are relatively more numerous in mid-pregnancy (approximately 6 per square centimetre) than at term (approximately 2.5 per square centimetre). Microscopically, the areolae have a different type of epithelium and vascular pattern from the interareolar area, details of which are described by Abromavich (1926); Goldstein, (1926); Heuser (1927); Brambell (1933); and Wislocki and Dempsey (1946b). The epithelial cells are mostly simple columnar. All have small darkly staining nuclei and a pale cytoplasm containing numerous vacuoles; the contents of which stain like the secretion (uterine milk) located in the areolar cups. The cells are further characterised by long lasting deposits of glycogen and dense concentrations of iron and acid phosphatase (Wislocki and Dempsey, 1946b). The blood supply to the folds in the wall of the cup comes from a capillary ring, linked with the capillaries in the interareolar area, and is drained by a number of venules depending on the number of making up the wall of the cup. In the areolae, the capillaries never displace the epithelial cells as they do in the chorionic ridges of the interareolar areas.”
In a photograph of this region from a ‘Loris’, Wooding and Flint (1994, p. 309) labeled the regions as ‘chorionic vesicles’ as did Amoroso when he considered a number of other species with similar structures. These authors showed them as being largely empty with short cristae emanating from the wall. Amoroso then went on to describe similar regions in a variety of animal placentas and dismissed their possible outcome as hippomanes in lemuroids, as had been speculated. The true function of areolae is presumably absorptive but it was speculated upon as yet unknown, according to Wooding and Flint.
This placenta of this animal measured 4 x 2.4 x 0.3 cm and weighed 1.4 g, and there were around 10 red-brown areolae in this placenta of a slender loris; each was between 0.2 and 0.4 cm in diameter.
5. Details of fetal/maternal barrier
This is a typical epitheliochorial villous placenta with the trophoblast being single-layered and presumably directly apposed to the uterine epithelium. Placentas from similar types of animals show that there is no true fusion of trophoblast with the maternal endometrial epithelium.
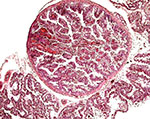
6. Umbilical cord
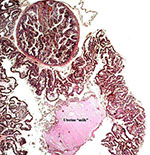
The umbilical cord was 4.5 cm long and only slightly twisted. It contained 3 blood vessels and an allantoic duct. The allantoic duct is lined by a flat epithelium and the cord’s surface (near the abdominal insertion) is covered with keratinized epithelium.
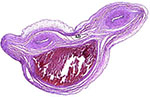 |
Cross section through the umbilical cord with two arteries and one vein, as well as an allantoic duct (SD). |
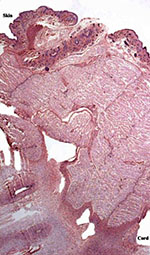 |
Insertion of the umbilical cord on the fetal abdomen. |
7. Uteroplacental circulation
No attached placenta has been available of this species and thus, the general circulation, other than the fetal circulation, cannot be described. The latter has been the topic of detailed description by Hill et al. (1928).
8. Extraplacental membranes
A large, lobed allantoic sac is present only some portions of which are vascularized. The amnion is avascular and has a thin squamous epithelium on delicate connective tissue.
9. Trophoblast external to barrier
None is known to exist as implanted placentas have not been seen.
10. Endometrium
The uterus is bicornuate and no decidua forms in pregnancy (Mossman, 1987).
11. Various Features
None exist so far as is known other than the descriptions of 3 embryos of N. lydekkerianus by Hill et al. (1928) which focuses much on the development of the vitelline and allantoic circulations as well as their membrane development.
12. Endocrinology
There are reports (cited by Mossman & Duke, 1973) that adult lorisidae have a fetal type of ovarian structure with continued oogenesis. Estrone was determined in fecal specimens of pygmy lorises and it was found to be useful in determining the number of offspring and the beginning of pregnancy termination; the authors also evaluated aromatase activity in their placentas (Jurke et al., 1997; 1998). The length of pregnancy was thus suggested to be 187-198 days for pygmy lorises.
The fetal adrenal gland of this animal had a wide ‘fetal zone’ and very thin definitive cortex as is shown in the attached picture.
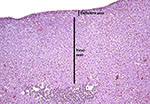 |
Fetal adrenal gland with medulla under left bar. |
13. Genetics
The slender loris has 62 chromosomes; samples are maintained in the “Frozen Zoo” at the Safari Park of the Sand Diego Zoo. Samples are available by contacting Dr. Oliver Ryder at oryder@sandiegozoo.org. The chromosomes are VERY different from those of the pygmy slow loris from Vietnam that has only 50 chromosomes (see data on that species in this volume). Molecular data (mtDNA) have been made available by Roos (2004) which justify the designation of three Nycticebus species. The evolution of growth hormone (GH) and prolactin was studied by Wallis et al. (2001; 2005); the results suggested rapid changes and lack of GH gene duplication in loris species. Similarly, prolactin had an ‘episodic pattern’ of evolution. Other genetic studies may be found in papers by Su et al. (1997) and Hacia et al. (1998).
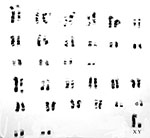 |
Giemsa-banded karyotype of a slender loris and originating from the San Diego Zoo (From O'Brien et al., 2006). |
14. Immunology
MHC class I cDNA sequences were delineated for a number of primates by Flügge et al. (2002).
15. Pathological features
This was a stillborn animal without pathological findings. Griner (1983) did not include any specimens in his review of animal pathology.
16. Physiologic data
Groves (2004) makes the point that ‘no taxonomic statements should be made on the basis of pelage characters’ because of the considerable variability (see Streicher, 2003; 2004). Ravosa (1998) provided results from cranial and dental measurements of the two species of loris and found the results to supports Groves’ original classification. An interesting study comes from Fisher et al. (2003) that suggests that a familiarity with the odor of males enhances breeding success. Fitch-Snyder & Ehrlich (2003) compared the mother-infant interactions between two different loris species and found them to be similar.
17. Other resources
Testes are said to descend seasonally into the scrotum (Parkes, 1956) and a penile baculum is present.
18. Other remarks – What additional information is needed?
Early implantational descriptions are badly needed, as is a mature placenta that is still attached to the uterus.
Acknowledgement
This specimen was kindly made available by the pathologists of the San Diego Zoo.
References
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall’s Physiology of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown & Co. Boston, 1961.
Enders, A.C. and Carter, A.M.: Comparative placentation: Some interesting modifications for histotrophic nutrition – a review. [This is a complicated reference]. Trophoblast Research 20:S1-S144, April 2006 – or: Placenta 27 Suppl. A, April 2006.
Fisher, H.S., Swaisgood, R.R. and Fitch-Snyder, H.: Odor familiarity and female preferences for males in a threatened primate, the pygmy loris Nycticebus pygmaeus: applications for genetic management of small populations. Naturwissenschaften 90:509-512, 2003.
Fitch-Snyder, H. and Ehrlich, A.: Mother-infant interactions in slow lorises (Nycticebus bengalensis) and pygmy lorises (Nycticebus pygmaeus). Folia Primatol. (Basel) 74:259-271, 2003.
Flügge, P., Zimmermann, E., Hughes, A.L. Günther, E. and Walter, L.: Characterization and phylogenetic relationship of prosimian MHC class I genes. J. Mol. Evol. 55:768-775, 2002.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Groves, C.: Primate Taxonomy. Smithsonian Institution Press, Washington, 2001.
Hacia, J.G., Makalowski, W., Edgemon, K., Erdos, M.R., Robbins, C.M., Fodor, S.P., Brody, L.C. and Collins, F.S.: Evolutionary sequence comparisons using high-density oligonucleotide arrays. Nature Genet. 18:155-158, 1998.
Hill, J.P., Ince, F.E. and Rau, A.S.: The development of the foetal membranes in Loris, with special reference to the mode of vascularisation of the chorion in the Lemuroides and its phylogenetic significance. Proc. Zool. Soc. London pp. 699-718, 1928.
Jurke, M.H., Czekala, N.M. and Fitch-Snyder, H.: Non-invasive detection and monitoring of estrus, pregnancy and the postpartum period in pygmy loris (Nycticebus pygmaeus). Amer. J. Primatol. 41:103-115, 1997.
Jurke, M.H., Czekala, N.M., Jurke, S., Hagey, L.R., Lance, V.A., Conley, A.J. and Fitch-Snyder, H.: Monitoring pregnancy in twinning pygmy loris (Nycticebus pygmaeus). Amer. J. Primatol. 46:173-183, 1998.
Kolar, K. and Wendt, H.: Spitzhörnchen und Halbaffen. Chapter 13 in Grzimeks Tierleben, Vol. 10, Säugetiere 1, Kindler Verlag AG, Zürich, Switzerland, 1967.
Manley, G.H.: Reproduction in lorisoid primates. Symp. Zool. Soc. London 15:493-509, 1966; and : Manley, G.H.: Gestation periods in the Lorisidae. Int. Zoo Ybk. 7:80-81, 1967.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nowak, R.M. and Paradiso, J.L.: Walker’s Mammals of the World, 4th ed. Vol. I. The Johns Hopkins University Press, Baltimore, 1983.
O’Brien, S.J., Menninger, J.C. and Nash, W.G., eds.: Atlas of Mammalian Chromosomes. Wiley-Liss; A. John Wiley & Sons, Inc. Hoboken, N.J., 2006.
Parkes, A.S.: Marshall’s Physiology of Reproduction, Vol. I. Longmans, Green and Co. London, 1956.
Ravosa, M.J.: Cranial allometry and geographic variation in slow lorises (Nycticebus). Amer. J. Primatol. 45:225-243, 1998, and 47:89, 1999.
Roos, C.: Molecular evolution and systematics of Vietnamese primates. Chapter 4 (pp.23-28) in Nadler, T., Streicher, U. and Long, H.T.: Conservation of Primates in Vietnam. Haki Publishing, Hanoi, 2004.
Streicher, U.: Seasonal changes in the colouration and fur patterns in the pygmy loris (Nycticebus pygmaeus). Chapter 5 (pp.29-32) in Nadler, T., Streicher, U. and Long, H.T.: Conservation of Primates in Vietnam. Haki Publishing, Hanoi, 2004.
Su, B., Wang, W., Lan, H. and Zhang, Y.: Genetic diversity and phylogenetic relationships among Chinese Macacas based on protein electrophoresis. Yi Chuan Xue Bao 24:109-115, 1997.
Wallis, O.C., Mac-Kwashie, A.O., Makri, G. and Wallis, M.: Molecular evolution of prolactin in primates. J. Mol. Evol. 60:606-614, 2005.
Wallis, O.C., Zhang, Y.P. and Wallis, M.: Molecular evolution of GH in primates: characterization of the GH genes from slow loris and marmoset defines an episode of rapid evolutionary change. J. Mol. Endocrinol. 26:236-258, 2001.
Wooding, F.B.P. and Flint, A.P.F.: Placentation. Chapter 4 (pp.233-460) in, G.E. Lamming, ed. Marshall’s Physiology of Reproduction, 4th ed. Vol. 3, Part 1. Chapman & Hall, London, 1994.