| |
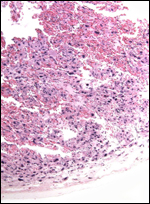
8) Extraplacental membranes
The "free membranes" are the bilaminar omphalopleure with thick
Reichert membrane. The external epithelium is not endodermal, but it is
the inner epithelium. Thus, this is not an inverted yolk sac placentation.
Instead the ectodermal epithelium fuses with the endometrium and here forms,
in essence, a syndesmochorial region. This membrane and the uterine wall
are very thin.
9)
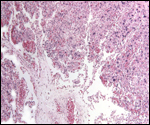
Trophoblast external to barrier
Only the decidual and vascular infiltration of trophoblast are seen external
to the placenta. This does not involve the uterine muscle.
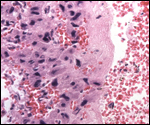
10) Endometrium
The decidua undergoes considerable changes during placental development.
This has been described in great detail by Fischer & Mossman (1969)
but does no seem to be important for this consideration of the placenta.
11)
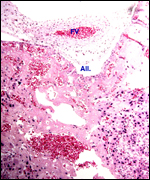
Various features
No true "sub-placenta" is present, but the early stages of development
produce a very large "pre-placenta", akin to former designations
"ectoplacenta". This consists then of pure trophoblastic epithelial
cells and leads to the development of maternal blood channels.
12)
Endocrinology
Merwe et al. (1980) found that the progesterone levels in pregnancy correlated
with the size of the corpus luteum (from 61 to 92 ng/ml) and were relatively
similar throughout gestation. Luteinizing hormone (LH) concentrations
were high in early pregnancy and declined later. The LH was measured by
a species-cross-reacting sheep antibody, but values were not given in
the publication. Estrogens were not reported.
Fischer & Mossman (1969) assumed that gonadotropins and steroids are
produced by trophoblast, as in other rodents. But they provided no evidence
for it and merely referred to an old paper by Deane et al. (1962).
13)
Genetics
Only one chromosomal study has been reported on these animals. We have studied a single male and female specimen and found its chromosome number to be 2n=38. The karyotype that was then published was done with an early Giemsa banding method (Bogart et al., 1976; Hsu & Benirschke, 1977) . It needs to be repeated. Hybrids with other species are unknown. Matthee & Robinson (1997 a,b ) studied mtDNA in an attempt to better place the species phylogenetically. Although their results are also not definitive, they placed the species with Heteromyidae, Geomyidae and Muridae in one clade, separating it from a clade containing the Hystricidae, Thryonomidae and Sciuridae. They suggested that this is consistent with the placental findings. In addition, however, they were able to delineate the karyotypes of these two distinct subspecies of springhare more extensively (Matthee & Robinson, 1997b). They found that the East African population ( P. c. surdaster ) had 2n=40 while the South African population ( P. c. capensis ) had 2n=38 with a single Robertsonian translocation (between acrocentrics ## 16 and 17) being the mechanism of this difference. The authors provide a map of the distribution of these two groups and suggested that: “Although the pronounced mitochondrial phylogeographical break, which characterizes the southern and east African springhare populations, is indicative of long-term historical population separation, there is little doubt that at some stage in their evolutionary past the species was continuously distributed between the two geographic isolates”. Finally they consider older taxonomic studies that separated Pedetes into separate species.
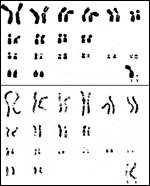 |
Karyotypes of Pedetes c. capensis from Hsu & Benirschke, 1977. |
14)
Immunology
No studies are known to me.
15)
Pathological features
A newly imported springhaas with widespread filariasis was described by
Anderson et al. (1998). The organism (Filaria versterae) is apparently
normally confined to subcutaneous tissue but in this animal, it invaded
into thoracic and peritoneal cavities. Because of pulmonary involvement,
the animal had to be euthanized. Butynski (1979) found many animals to
be infected with a stomach nematode (Physaloptera capensis) that
did not have any apparent deleterious impact.
16)
Physiologic data
An interesting study on osmoregulation was done by Peinke & Brown
(1999). Since the animal is exposed to very arid conditions, they deprived
animals of water for up to seven days to test osmotic regulation. They
lost up to 30% body weight during that period and regulated their hematocrits
and electrolytes appropriately. They thus maintained their plasma volume
despite the water deprivation by producing concentrated urine and reducing
fecal water loss.
17)
Other resources
Many animals exist in zoological gardens, but breeding colonies are not
known to exist. We have no cell lines for study in our collection of cell
strains at CRES.
18)
Other remarks - What additional Information is needed?
Because of the great complexity of the organ, additional studies are needed.
It will be of interest to know the length of the umbilical cord, the number
of cord blood vessels, and actual data on gonadotropin and steroid production
with cellular localization. Chromosomal studies with comparing the results
to other rodents are desirable.
Acknowledgement
Most of the animal photographs in these chapters come from the Zoological
Society of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo. I am appreciative for the material obtained from
Dennis Meritt, Ph.D.
References
Anderson, R.C., Gustafson, B.W. and Williams, E.S.: Acute filariasis in
a springhaas. J. Wildl. Dis. 34:145-149, 1998.
Bogart,
M.H., Scollay, P.A., Cooper, R.W. and Benirschke, K.: Springhaas (Pedetes
capensis). Chromos. Inform. Serv. 30:14-15, 1976.
Butynski,
T.M.: Reproductive ecology of the springhaas Pedetes capensis in
Botswana. J. Zool. (London) 189:221-232, 1979.
Coe,
M.J.: The anatomy of the reproductive tract and breeding in the spring
haas, Pedetes surdaster larvalis Hollister. J. Reprod. Fertil.
Suppl. 6, 159-174, 1969.
Deane,
H.W., Rubin, B.L., Driks, E.C., Lobel, B.L. and Leipsner, G.: Trophoblast
giant cells in placenta of rats and mice and their probable role in steroid
hormone production. Endocrinology 70:407-419, 1962.
Fischer,
T.V. and Mossman, H.W.: The fetal membranes of Pedetes capensis,
and their taxonomic relevance. Amer. J. Anat. 124:89-116, 1969.
Gotch,
A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset,
1979.
Hediger,
H. Gefangenschaftsgeburt eines afrikanischen Springahasen, Pedetes caffer.
Zool. Garten 17:166-169, 1950.
Horst,
C.J. v.d.: On the reproduction of the springhare, Pedetes caffer.
Pamph. S. Afr. Boil. Soc. 8:47, 1935.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 10, Folio 456, 1977.
Matthee, C.A. and Robinson, T.J.: Molecular phylogeny of the springhare, Pedetes capensis , based on mitochondrial DNA sequences. Mol. Biol. Evol. 14:20-29, 1997a.
Matthee , C.A. and Robinson, T.J.: Mitochondrial DNA phylogeography and comparative cytogenetics of the springhare, Pedetes capensis (Mammalia: Rodentia). J. Mammalian Evol. 4:53-73, 1997b.
Merwe,
M.v.d.. Skinner, J.D. and Millar, R.P.: Annual reproductive pattern in
the springhaas, Pedetes capensis. J. Reprod. Fertil. 58:259-266, 1980.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Otiang'a-Owiti,
G.E., Oduor-Okelo, D. and Gombe, S.G.: Foetal membranes and placenta of
the springhare (Pedetes capensis larvalis Hollister). Afr. J. Ecol.
30:74-86, 1992.
Owiti,
G.E.O., Oduor-Okelo, D. and Gombe, S.: Ultrastructure of the chorioallantoic
placenta of the springhare Pedetes capensis larvalis Hollister.
Afr. J. Ecol. 23:145-152, 1985.
Peinke,
D.M. and Brown, C.R.: Osmoregulation and water balance in the springhare
(Pedetes capensis). J. Comp. Physiol. [B] 169:1-10, 1999.
Puschmann,
W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag
Berlin, 1989.
Rosenthal,
M.A. and Meritt, D.A.: Hand-rearing springhaas Pedetes capensis
at Lincoln Park Zoo, Chicago. Intern. Zoo Yearb. 13:135-137, 1973.
Simpson.
G.G.: The principles of classification and a classification of mammals.
Bull. Amer. Museum Nat. Hist. 85:1-350, 1945.
|