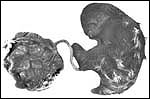
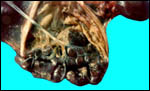
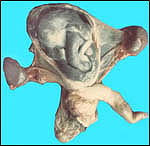
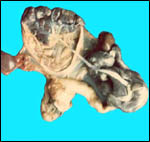
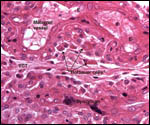
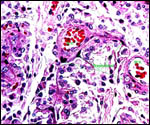
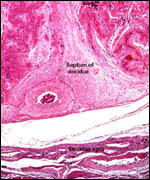
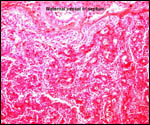
| |
13)
Genetics
Personal communications from Dr. W. Jorge in Brazil indicates that all five
specimens studied by him had 54 chromosomes, with an XX/XY sex determining
mechanism. The same author, however, provided additional details in his
later contribution (Jorge et al., 1985). B. tridactylus had 52 elements,
B. infuscatus (probably variegatus) had 54 chromosomes, and a specimen
of B. variegatus had 55 chromosomes. In a later contribution, Jorge
& Pinder (1990) studied the very much endangered Bradypus torquatus
from Northern Brazil and found them to have 2n=50, with XX/XY sex determining
mechanism.
No hybrids have been described.
14)
Immunology
I know of no immunological studies done in sloths.
15)
Pathological features
Diniz and Oliveira (1999) described the clinical and pathological conditions
of sloths in captivity. They found digestive and respiratory problems
to be the most notable. A variety of parasites were found in fecal examinations
and they stated that the first 6 months in captivity were the most critical.
Thereafter, management is less problematic. Seymour et al. (1983) isolated
a large number of poorly studied viruses from sloths in Panama. Further
details on parasites infecting the sloths may be found in contributions
to the monograph by Montgomery (1985).
16)
Physiologic data
A variety of physiologic studies have been undertaken in Bradypus.
For instance, da Mota et al. (1992) have studied pancreatic endocrine
cells and found that glucagon-producing cells are much commoner than found
in the pancreas of human or laboratory animals. They related this to their
folivorous diet and possibly to their phylogeny. A comprehensive review
of sloths' physiology is found in the contribution by Gilmore et al. (2000).
In their additional paper of 2001, these authors specifically elaborated
on the hair structure of sloths with its algal growth, as well as their
ecology of carrying specific disease agents (viruses, parasites, and leishmania).
The cardiovascular adaptation to postural change was studied by Duarte
et al. (1982). Many other fascinating details of their physiology are
highlighted in an editorial (1974), such as the abdominal testes, the
conservation of temperature, the infrequent urination and defecation.
17)
Other resources
Some cell strains are available from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder at: oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
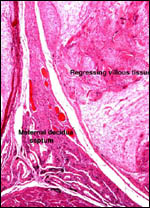
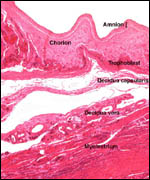
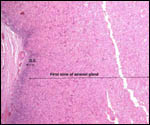
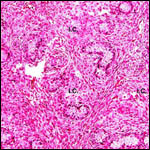
Endocrine studies of placentas and gestation are needed to identify the
possible presence or the absence of placental gonadotropins. The presence
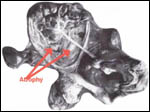
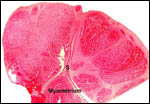
of so large a fetal adrenal gland demands the study of fetal endocrine
aspects.
Acknowledgement
I appreciate very much the help of the pathologists at the San Diego Zoo.
References
Becher, H.: Zur Kenntnis der Placenta vom Bradypus tridactylus.
Z. Anat. Entwicklungsgesch. 61:114-136, 1921.
Benirschke,
K. and Powell, H.C.: On the placentation of sloths. Pp. 237-241, In, Montgomery,
G.G., ed.: The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas.
Smithsonian Institution, Washington, 1985.
Delsuc,
F., Catzeflis, F.M., Stanhope, M.J. and Douzery, E.J.: The evolution of
armadillos, anteaters and sloths depicted by nuclear and mitochondrial
phylogenies: implications for the status of the enigmatic fossil Eurotamandua.
Proc. Roy. Soc. Lond. B Biol. 268:1605-1615, 2001.
Diniz,
L.S. and Oliveira, P.M.: Clinical problems of sloths (Bradypus sp.
and Choloepus sp.) in captivity. J. Zoo Wildl. Med. 30:76-80, 1999.
Duarte,
D.P., da Costa, C.P. and Huggins, S.E.: The effects of posture on blood
pressure and heart rate in the three-toed sloth, Bradypus tridactylus.
Comp. Biochem. Physiol. A 73:697-702, 1982.
Editorial:
Sloth's compensations. The Lancet ii:1054, 1974.
Gilmore,
D.P., da-Costa, C.P. and Duarte, D.P.: An update on the physiology of
two- and three-toed sloths. Braz. J. Med. Biol. Res. 33:129-146, 2000.
Gilmore,
D.P., da Costa, C.P. and Duarte, D.P.: Sloth biology: an update on their
physiological ecology, behavior and role as vectors of arthropods and
arboviruses. Braz. J. Med. Biol. Res. 34:9-25, 2001.
Greenwood,
A.D., Castresana, J., Feldmaier-Fuchs, G. and Paabö, S.: A molecular
phylogeny of two extinct sloths. Mol. Phylogenet. Evol. 18:94-103, 2001.
Heuser,
C.H. and Wislocki, G.B.: Early development of the sloth (Bradypus griseus)
and its similarity to that of man. Contrib. Embryol. Carnegie Inst. Washington
25:1-13, 1935.
Hoke,
J.: Oh, it's so nice to have a sloth around the house. Smithsonian 18:88-98,
1987.
Jorge,
W., Orsi-Souza, A.T. and Best, R.: The somatic chromosomes of Xenarthra.
Pp.121 in, The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas.
G.G. Montgomery, ed. Smithsonian Institution, Washington, 1985.
Jorge,
W. and Pinder, L.: Chromosome study on the maned sloth Bradypus torquatus
(Bradypodidae, Xenarthra). Cytobios 62:21-25, 1990.
King,
B.F., Pinheiro, P.B.N. and Hunter, R.L.: The fine structure of the placental
labyrinth in the sloth, Bradypus tridactylus. Anat. Rec. 202:15-22,
1982.
Lange,
D.de: Quelques remarques sur la placentation de "Bradypus".
Compt. Rend. Assoc. Anat. Liège 21:321-333, 1926.
Meritt,
D.A. and Meritt, G.F.: Sex ratios of Hoffmann's sloth, Choloepus hoffmanni
Peters, and three-toed sloth, Bradypus infuscatus Wagler, in Panama.
Amer. Midl. Nat. 96:472-473, 1976.
Montgomery,
G.G., ed.: The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas.
Smithsonian Institution, Washington, 1985.
Moser,
H.G. and Benirschke, K.: The fetal zone of the adrenal gland in the nine-banded
armadillo, Dasypus novemcinctus. Anat. Rec. 143:47-60, 1962.
Da
Mota, D.L., Yamada, J., Gerge, L.L. and Pinheiro, P.B.: An immunohistochemical
study on the pancreatic endocrine cells of the three-toed sloth, Bradypus
variegates. Arch. Histol. Cytol. 55:203-209, 1992.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Seymour,
C., Peralta, P.H. and Montgomery, G.G.: Viruses isolated from Panamanian
sloths. Amer. J. Trop. Med. Hyg. 32:1435-1444, 1983.
Wetzel,
R.M. and Avila-Pires, D.D. de: Identification and distribution of the
recent sloths of Brazil (Edentata). Rev. Bras. Biol. 40:831-836, 1980.
Wetzel,
R.M. and Kock, D.: The identity of Bradypus variegatus Schinz (Mammalia,
Edentata) Proc. Biol. Soc. Wash. 86:25-34, 1973.
Wetzel,
R.M.: Systematics, distribution, ecology, and conservation of South American
edentates. Pp. 345-375, in "Mammalian Biology in South America. Special
Publication Series of the Pymatuning Laboratory of Ecology, University
of Pittsburg, Vol. 6, 1982.
Wetzel,
R.M.: The identification and distribution of recent Xenarthra (=Edentata).
Pp. 5-21, In, Montgomery, G.G., ed.: The Evolution and Ecology of Armadillos,
Sloths, and Vermilinguas. Smithsonian Institution, Washington, 1985.
Wislocki,
G.B.: On the placentation of the sloth (Bradypus griseus). Contrib.
Embryol. Carnegie Inst. Washington 16:7-19, 1925.
Wislocki,
G.B.: Further observations upon the placentation of the sloth (Bradypus
griseus). Anat. Rec. 32:45-51, 1926.
Wislocki,
G.B.: On the placentation of the tridactyl sloth (Bradypus griseus)
with a description of some characters of the fetus. Contrib. Embryol.
Carnegie Inst. Washington 19:211-228, 1927.
Wislocki,
G.B.: Further observations upon the minute structure of the labyrinth
in the placenta of the sloths. Anat. Rec. 40:385-395, 1928.
|