Melursus ursinus(Ursus ursinus)
Order: Carnivora
Family: Ursidae
1) General Zoological Data
This single Asiatic bear (India, Sri Lanka and neighboring countries) weighs between 55 and 145 kg (Nowak, 1999). It is mainly nocturnal and termites are its most important food source. The bear is characterized by his long, shaggy hair, the white V-shaped mark on the chest, and unusual lips that earn this species the name “Lippenbär” (lip bear). Longevity in zoos is up to 40 years. It is now endangered, primarily because its bile and other body parts are being used “medicinally”. The term “Melursus” has been used at times and refers to the honey eaten by these bears in conjunction with ants and bees. The usage of “sloth” refers to its ”slow, shambling gait” (Gotch, 1979). Many sloth bears are found in zoos and a “SSP” exists for this species to monitor its status in zoos.
There is still considerable debate on the evolution of bears although Thenius & Hofer (1960) considered it to have been essentially solved. It is believed that the animals derive from a fox-sized ancestor (Ursavus) in late Miocene. Sloth bear and sun bear are currently the most “primitive” bears. Some authors, as Nash & O’Brien (1987) and Nash et al. (1998) have the root to be a primitive carnivore with low chromosome number that subsequently split to arrive at the ursid 2n=74. Zhang & Ryder (1994) also discussed the ursid tree and studied mtDNA of all species and pandas. In their view, the spectacled bear split first, to be followed by sloth bear, the other following later.
 |
Sloth bear with two young at National Zoo, Washington, D.C. (Courtesy, rights@fonz.org) |
2) General Gestational Data
Gestation is said to last 6-7 months (Nowak, 1999), but this does not take into account that most (probably all) bears have a long period of delayed implantation. Generally, bears den up in winter and emerge with newborns. Thus, the placenta is usually not available and few records are available of the actual birth (see, however, Dittrich & Einsiedel, 1961). Essentially nothing is known about the precise circumstances of early placental development and what triggers implantation, but Wimsatt (1963) who observed 7 black bear uteri during preimplantation stages concluded that the blastocysts did not develop significantly in the months of quiescence. He also found the zona pellucida to be very thick and observed possible MZ twinning in one. There is only one pertinent report of the preimplantation stage in a sloth bear (Puschmann et al., 1977). These authors found free blastocysts in the uteri of sloth bears in September and freshly implanting blastocysts in November. Since most bears in European zoos deliver in March, these authors deduced an implantation length of 8-10 weeks, with one or two young, rarely three being born. Four are truly exceptional, but have been recorded. Other free blastocysts were discovered in black bears (Kronenberger, 1964). They have been found in the right and left horns of the uterus. Unfortunately, most bears consume the placenta immediately after parturition and therefore not much is known of their anatomy. Newborns weigh 300-500 g. Because of the precarious status of this species and that of some other bears, Johnston et al. (1994) have begun to collect oocytes from bears. 40% of those recovered from the sloth bear were in excellent condition.
3) Implantation
There is delayed implantation but few details are known because of the cryptic state of bear pregnancies.
4) General Characterization of the Placenta
Mossman (1987) has an interesting statement on the shape of the bear placenta that is worth repeating here: “Their membranes… are so broad antimesometrially and taper so rapidly laterally that those of the European brown, American black, and spectacled bears were considered discoid by Rau (1925), Wimsatt (1974), and Michel (1984), respectively. My observations on the membranes of a black bear and a few grizzlies, together with Young’s (1969) illustration of the conceptus of a polar bear, convince me that the placentas would be more appropriately described as broad interrupted zonary ones.”
Michel et al. (1983) described the structure of the placenta in a spectacled bear (Tremarctos ornatus). That placenta was discoid (12 x 9.5 x 0.5 cm) and weighed 55.4 g. At its margin was a circumferential reddish “marginal hematoma”. The structure is similar to that of the sloth bear shown below but this placenta had a decidua basalis with remnants of uterine glands. The photographs accompanying that report suggest that the umbilical cord (not described) had a membranous insertion. The late Prof. Elze kindly sent me those photographs and they are shown next because so few macroscopic placentas from any bear species are available...
 |
Gross appearance of the Spectacled bear placenta of Michel et al. (1983). Left maternal surface, right fetal surface. |
The placenta of a brown bear (Ursus arctos) was described in some detail by Rau (1925). It was also discoid and had a circumferential hematoma. Four allantochorial vessels “passed from the extraplacental allanto-chorion to the free surface of the placenta” (similar to the picture shown by Michel et al. (1983) in the sloth bear. The placenta is slightly lobulated, similar to that of the dog. Young (1969) described slightly more detail of the placenta of a polar bear. It appears as though this placenta was nearly zonary, occupying both uterine horns and the uterine body, measuring 10 cm in width and 20 cm in circumference, 0.75 cm in thickness.
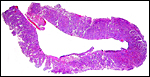 |
Complete cross section of the bear placenta. |
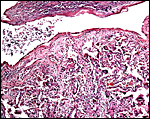
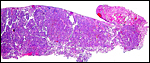 |
Edge of the immature placenta of sloth bear showing marginal necrotic material, not containing much blood, however. |
5) Details of fetal/maternal barrier
The placenta I have had for study came from a Ceylonese sloth bear that aborted a near-term stillborn fetus of 382 g and 20 cm crown-rump length. The male fetus was born in January; it still had some active glomerulopoiesis and was thus judged to be slightly immature. There was not only placental infection but the stomach of the fetus had colonies of bacteria within the mucus. Remarkably, there were no organisms in the lung.
This immature 10 x 1 cm placenta had no umbilical cord attached. It is a labyrinthine placenta with endotheliochorial characteristics but the fact that some villi protruded into the marginal hematomas has suggested to Michel (1983) that, in places, the bear placenta is a hemochorial organ.
Young (1969), on the other hand, described the polar bear placenta as being mostly syndesmochorial although at the edge some villi infiltrated an orange-colored region.
6) Umbilical cord
I cannot find records of the umbilical cord length of any bear. Young (1969), however, at least described that the cord of a polar bear contained two arteries and two veins, in addition to an allantoic duct. The veins fused near the fetal surface, the arteries fused near the placental surface. In addition there were minor vitelline vessels.
7) Uteroplacental circulation
This has not been investigated.
8) Extraplacental membranes
The allantoic sac is very large. Young (1969) described for the polar bear placenta that the yolk sac measured 6 x 1 cm, and that amnion and allantoic sacs were applied to the chorionic sac. The chorion had numerous villous processes, even more so at the margins.
9) Trophoblast external to barrier
To the best of my knowledge, uterine invasion of trophoblast does not occur. This placenta showed foci of detached decidua; Michel et al. (1983) depicted a thick “basal glandular zone” in the placenta of their spectacled bear.
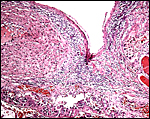
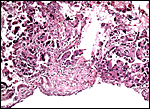 |
Base of the sloth placenta with remnants of decidua. |
10) Endometrium
The uterus is bicornuate with a short body. A thick basal decidua is detached with the placenta.
11) Various features
Wimsatt (1963) suggested from his observations in black bears that ovulation is induced. There is no subplacenta.
12) Endocrinology
The reproductive cycles, ovulation, estrus, etc. have all been studied and are summarized by Wimsatt (1963) in his large paper on delayed implantation. Corpora lutea extend throughout pregnancy.
13) Genetics
Hard (1968) was the first author to study the chromosomes of a female sloth bear; he found 2n=74. The comparative karyotypes of the Malayan sun bear, and some “related species”, have recently been studied in some detail by Tian et al. (2004). As previous investigators, they found all ursidae (except the South American spectacled bear with 2n=52) to possess 74 chromosomes, as was also published by Nash & O’Brien, 1987 and Nash et al., 1998). Much more difficult (for me) to accept is their notion that this ursid karyotype (presumably ancestral for ursidae) derived from a putative “ancestral carnivore karyotype” (2n=42 or 44 as some of the papers suggest) and that the bear karyotype came about, as they have postulated, by numerous fissions and one inversion.
Hybrids among bears are common and, when allowed to grow up, they are mostly fertile (see Kowalska, 1969 and Wurster-Hill & Bush, 1980). The sloth bear was listed to have made hybrids with a Malayan sun bear in Tokyo, and with an Asiatic black bear (Selenarctos thibetanus) (Gray, 1972; Asakura, 1969; Scherren, 1907). The fertility of the offspring is not detailed.
 |
Karyotype of male sloth bear from the San Diego Zoo. |
 |
Karyotype of female sloth bear from the San Diego Zoo. |
14) Immunology
I know of no studies.
15) Pathological features
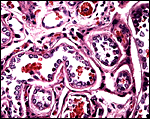
Griner (1983) performed a single autopsy in an adult female sloth bear. It had died from acute enteritis and there was a suspicion of panleukopenia. The specimen available to me had intraplacental abscesses and surface inflammation (“chorionitis”), in addition to marked inflammation of the umbilical blood vessels. The immature male fetus had colonies of bacteria in his stomach, obviously ingested prior to delivery. Remarkably, the lung was free of organisms.
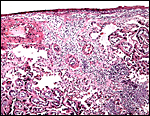 |
Acute chorionitis, abscesses in the villous tissue. |
Black bears often have significantly enlarged baculae, which are at time ossified and simulate a penis, without detriment to their fertility, as placental scars were found in the uteri; the cause of this masculinization is unknown and is apparently not due to ovarian factors (Cattet, 1988).
16) Physiologic data
Hagey et al. (1993) made the interesting observation that all bears have ursodeoxycholic acid (UDCA) as their primary cholic acid, and that in all this is conjugated with taurine. No panda, raccoon or other carnivore possessed these compounds. Moreover, sloth bear and sun bear had only small amounts (as did the spectacled bear), compared to much larger percentages in the other bears. They suggested from these findings that the sloth bear is more “ancient”, and that the production of UDCA is a relatively recent evolutionary phenomenon.
The uterine/endometrial structure has been described in detail by Wimsatt (1963). It includes histochemical observations during the cycle and preimplantation period.
Bush et al. (1980) described anesthesia and provided chemical as well as hematological values in a variety of bears, including the sloth bear. Page (1986) used ketamine and xylazine for immobilization. The genital tract of pregnant and nonpregnant bears is readily accessible to sonographic evaluation (Goritz et al., 1997). Simultaneous to the sonographic study, progesterone values were obtained from feces; they had not risen in early gestation.
Lang (1943) described an unusual stereotypy of a pair of sloth bears at Basel Zoo; the vomiting and immediate eating of the vomited food was pronounced in one animal.
17) Other resources
Several strains of fibroblast cells are deposited in the “Frozen Zoo” at CRES and can be made available by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other remarks – What additional Information is needed?
Most importantly, early stages of implantation are missing; the length and nature of the umbilical cord is unknown. Endocrine studies are largely missing.
Acknowledgement
The animal photograph in this chapter comes from the National Zoo, FONZ Website on Sloth Bear, with kind permission by FONZ. The karyotypes were kindly made available by Suellen Charter of CRES at San Diego Zoo.
References
Asakura, S.: A note on a bear hybrid, Melursus ursinus x Helarctos malayanus, at Tama Zoo, Tokyo. Int. Zoo Ybk. 9:88, 1969.
Bush, M., Custer, R.S. and Smith, E.E.: Use of dissociative anesthetics for immobilization of captive bears; blood gas, hematology and biochemistry values. J. Wildl. Dis. 16:481-489, 1980.
Cattet, M.: Abnormal sexual differentiation in black bears (Ursus americanus) and brown bears (Ursus arctos). J. Mammal. 69:849-852, 1988.
Dittrich, L. and Einsiedel, I.v.: Bemerkungen zur Fortpflanzungs und Jugendentwicklung des Braunbären (Ursus arctos L.) im Leipziger Zoo. Zool. Garten NF 25:250-269, 1961.
Goritz, F., Hildebrandt, T., Jewgenow, K., Wagner, N., Hermes, R., Strauss, G. and Meyer, H.H.: Transrectal ultrasonographic examination of the female urogenital tract in nonpregnant and pregnant captive bears (Ursidae). J. Reprod. Fertil. 51:303-312, 1997.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2 nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hagey, L.R., Crombie, D.L., Espinosa, E., Carey, M.C., Igimi, H. and Hofmann, A.F.: Ursodeoxycholic acid in the ursidae: biliary bile acids of bears, pandas, and related carnivores. J. Lipid Res. 34:1911-1917, 1993.
Hard, W.L.: The karyotype of a female sloth bear, Melursus ursinus. Mammal. Chromos. Newsl. 9:242-243, 1968.
Johnston, L.A., Donoghue, A.M., Igo, W., Simmons, L.G., Wildt, D.E. and Rieffenberger, J.: Oocyte recovery and maturation in the American black bear (Ursus americanus): a model for endangered ursids. J. Exp. Zool. 269:53-61, 1994.
Kowalska, Z.: A note on bear hybrids Thalarctos maritimus x Ursus arctos at Lodz Zoo. Intern. Zoo Ybk. 9:89, 1969.
Kronenberger, H.: Beitrag zur Fortpflanzungsbiologie des Baribal (Ursus americanus Pall.). Milu 1:298-302, 1964.
Lang, E.M.: Eine ungewöhnliche Sterotypie bei einem Lippenbären (Melursus ursinus Shaw). Schweiz. Arch. Tierheilk. 85:477-481, 1943.
Michel, G., Elze, K. and Seifert, S.: Zur Embryonalentwicklung des Bären unter besonderer Beachtung des Baues der Plazenta. Zool. Garten NF 53:290-294, 1983.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nash, W.G. and O’Brien, S.J.: A comparative banding analysis of the Ursidae and their relationship to other carnivores. Cytogenet. Cell Genet. 45:206-212, 1987.
Nash, W.G., Wienberg, J., Ferguson-Smith, M.A., Menninger, J.C. and O’Brien, S.J.: Comparative genomics: tracking chromosome evolution in the family Ursidae using reciprocal chromosome painting. Cytogenet. Cell Genet. 83:182-192, 1998.
Nowak, R.M.: Walker’s Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Page, C.D.: Sloth bear immobilization with ketamine-xylazine combination: reversal with yohimbine. J. Amer. Vet. Med. Ass. 189:1050-1051, 1986.
Puschmann, W., Schüppel, K.-F. and Kronberger, H.: Blastocystennachweis im Uteruslumen des Indischen Lippenbären, Verhandlungsbericht XIX. Internat. Sympos. Erkrankungen Zootiere ( Poznan) pp. 389-391, 1977. Published by the Tierpark Berlin.
Rau, A.S.: Contributions to our knowledge of the structure of the placenta of mustelidae, ursidae, and sciuridae. Proc. Zool. Soc. London 25:1027-1070, 1925.
Scherren, H.: Some notes on hybrid bears. Proc. Zool. Soc. London 431-435, 1907.
Thenius, E. and Hofer, H.: Stammesgeschichte der Säugetiere. Eine Uebersicht über Tatsachen und Probleme der Evolution der Säugetiere. Springer-Verlag, Berlin, 1960.
Tian, Y., Nie, W., Wang, J., Ferguson-Smith, M.A. and Yang, F.: Chromosome evolution in bears: reconstructing phylogenetic relationships by cross-species chromosome painting. Chromosome Res. 12:55-63, 2004.
Wimsatt, W.A.: Delayed implantation in the Ursidae, with particular reference to the black bear (Ursus americanus Pallas). Pp. 49-76, in: Delayed Implantation, A.C. Enders, ed., University of Chicago Pres, 1963.
Wurster-Hill, D.H. and Bush, M.: The interrelationship of chromosome banding patterns in the giant panda (Ailuropoda melanoleuca), hybrid bear (Ursus middendorfi x Thalarctos maritimus), and other carnivores. Cytogenet. Cell Genet. 27:147-154, 1980.
Young, A.: The foetal membranes of polar bear (Thalarctos maritimus). J. Anat. 104:200, 1969.
Zhang, Y.-P. and Ryder, O.A.: Phylogenetic relationships of bear (the Ursidae) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 3:351-359, 1994.