|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
| Last updated: May 20, 2004. |
Tragelaphus spekei
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
"Sitatungas of Africa are semiaquatic, spending most of their life in dense beds of Papyrus" (Nowak1999). Cotton (1935) also refers to their aquatic habitat and the fact that the animals are difficult to spot in the deep papyrus stands. Males are darker and larger than lighter brown females, and only the male has (spiraled) horns. The sitatunga is still widely distributed in East Africa but is said to have nearly disappeared from the west of Africa. Many animals are held in zoological gardens. Gotch (1979) indicated that the name sitatunga derives from an old Bantu language; tragos is Greek for male goat, and elaphos is a deer. Several regional subspecies have been nominated but not further delineated genetically.
The evolution of the tribe Tragelaphini has been of interest in general but to students of cytogenetics specifically. Most of their chromosome numbers are low (around 30) and they possess fusions of sex chromosomes with one specific autosome. The nyala is an exception with a much higher chromosome number (55-56). Wallace (1976, 1978) suggested that the nyala split of earliest, to be followed by speciation of the remainder of Tragelaphini. This, he suggested, was followed and enhanced by chromosomal fusions (see section on genetics below). Vrba & Schaller (2000) indicated that the first recognized tragelaphines are to be found in deposits of 6.5 MYA, having split from the bovid ancestors that are first seen 17 MYA. Matthee & Robinson (1999) studied the phylogeny with cytochrome b analysis of this tribe and came to the conclusion that all members should be assigned the genus Tragelaphus. They grouped nyala and sitatunga closely and suggested, from molecular time clock data, that these two species separated 9.5 MYA (!). Additional relevant studies by Matthee & Davis (2001) and Hassanin & Douzery (1999) provide little further evolutionary insight.
 |
East African Sitatunga at San Diego Zoo. |
2) General Gestational Data
Gestation lasts 240 and 250 days, as indicated above with usually singletons born. Neonates weigh around 4 kg and twins are uncommon.
3)
Implantation
Early implantational stages have not been described and little is known
of timing and manner of early placental formation.
4)
General Characterization of the Placenta
I have had the opportunity to study two placentas. One was from a normal
term delivery of a living offspring. It weighed 350 g and had only 28
very thin cotyledons that measured between 8 and 5 cm in diameters. The
implanted cotyledon, however, was 1.5 cm in maximal thickness. The umbilical
cord was 10 cm long.
The other placenta was attached to the uterus following Cesarean section
of a term fetus. The pregnancy was accompanied by "hydroallantois"
and remained attached to the uterus; the dam died during anesthesia and
following Cesarean section surgery with pleural adhesions and marked splenic
hemosiderosis. The fetus weighed 3.9 kg and was normally matured. The
allantois was hugely distended with a very small amnionic cavity. The
cord measured 10 cm in length, as the previously described cord, but there
were approximately 40 cotyledons present in this implanted placenta. The
female neonate also expired. The placenta had four rows of cotyledons.
The general structure of the cotyledons is similar to that described for
eland and bongo (Hradecky et al., 1987) although the number of cotyledons
enumerated for eland (142, 151) and bongo (155) by Hradecky (1983) was
very much smaller (28, 40). In the chapter on bongo of this web site the
placentas are also recorded as having many more cotyledons than was true
for the present species.
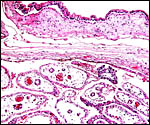
An additional placenta was received in May, 2004 and was still partially attached to a stillborn (2.45 kg); it is depicted next. This placenta had 25 large, flat cotyledons and weighed 460 g. It was similar in all respects to that of the previously described specimens.
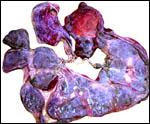 |
Sitatunga placenta from stillborn fetus. |
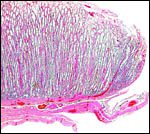 |
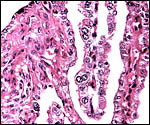
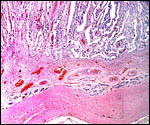
Margin of implanted cotyledon with myometrium below. |
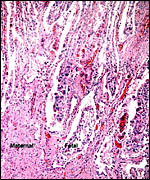
This is a typical multicotyledonary placenta with epitheliochorial contact to the uterus. There is much of the usual yellow-brown pigment accumulated beneath the chorionic plate of the cotyledons. The nature of the pigment has been discussed in various chapters on other ungulates of this web site. While it has been suggested to have a derivation from degenerating blood, it is always iron-stain-negative. I have suggested that it may be melanin deposits.
The villous surface is covered by a single layer of trophoblast among which are numerous binucleate cells. The trophoblast has intimate contact with the endometrial epithelium which is, again, single-layered. The following photographs show this well. The villi have central capillaries and scant stroma and, in my attached placenta shown, they are rather more edematous appearing beneath the chorionic plate than near the maternal aspect. They become more slender at that point.
The origin of binucleate cells that are so characteristic of ruminant placentas has been studied by numerous investigators, primarily in the sheep and cow. They are believed to produce a variety of glycoproteins, especially placental lactogen and Wooding (1982; Wooding et al., 1997) suggested that they arise by fusion of trophoblast with endometrial cells.
Both umbilical cords were 10 cm long, unspiraled and without plaques on their surfaces. They had four large blood vessels and numerous small allantoic vessels. There was a central allantoic duct. The cord of the third placenta, from a stillborn infant that had the placenta still attached measured 11 cm and was also unspiraled.
7)
Uteroplacental circulation
I am not aware of any published studies of the vascular aspects of the
bicornuate uterus in this species.
8)
Extraplacental membranes
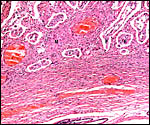
The amnion is rather smaller than the allantoic sac and is made of a single
layer of flat squamous epithelium on a sheet of connective tissue that
is without blood vessels. The allantoic connective tissue contains an
abundant circulation and its epithelium is somewhat more prominent and,
focally at least, multi-layered. I did not find remnants of the yolk sac
in these two placentas.
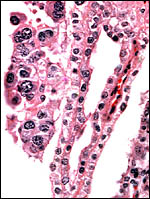
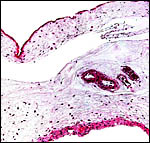 |
Allanto-amnionic membranes. The vascularized allantois is below, the amnion above. |
No trophoblastic invasion of the uterus was found in the one placenta I was able to study and that was attached to the uterus.
10)
Endometrium
There are four rows of endometrial caruncles that are easily identified
in the newborn uterus. The uterus is essentially bicornuate like in bovines,
and not "duplex" (Hradecky, 1982). There is no classical decidua.
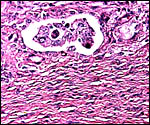
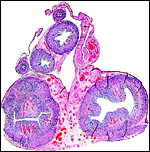 |
Cross sections through neonatal uterine horns. |
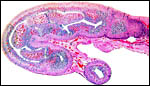 |
Longitudinal section of neonatal uterine horn. |
No other remarkable features were identified.
12) Endocrinology
The best-studied endocrine aspect of most ungulate species is the production of a variety of hormones by the binucleate trophoblast. Atkinson et al. (1993) identified a novel carbohydrate as originating from these cells and reviewed other publications that identify other glycoprotein hormones in these cells. It would appear that different populations of binucleate cells may exist with different end-products. Generally speaking, however, the cells are seen as producing primarily placental lactogen (Wooding, 1997).
No other endocrine studies of this species are known to me.
13)
Genetics
Sitatungas have 30 chromosomes (Koulischer, Tijkens, & Mortelmans,
1967; Wurster & Benirschke, 1968). The sex chromosomes are unusually
large - 13.08% for the X-, and 7.29% for the Y-chromosome (Wurster et
al., 1968). The entire Tribe is characterized by a translocation (fusion)
of the Y chromosome with an autosome (#13) (Petit et al., 1994). In addition,
some members also have an autosome/X chromosome fusion (Wallace, 1976).
The exception to the low chromosome number (around 30) is the nyala with
55 chromosomes (Wallace, 1978). Wallace also reviewed the possible evolution
of these animals from a possible ancestor with high chromosome number
and fusions following during speciation. My interpretation is depicted
below as a diagram. A fertile hybrid between Bongo and Sitatunga was described
by Koulischer et al. (1973). Other hybrids among tragelaphines are listed
by Gray (1972) without further description of their possible fertility.
The molecular studies on tragelaphines have been cited at the beginning
of this chapter. They place nyala and sitatunga most closely.
 |
Karyotype of male sitatunga. |
 |
Karyotype of female sitatunga. |
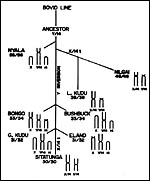 |
My interpretation of the phylogenetic origins of tragelaphine species. |
I know of no publications on immunological studies of sitatunga immunology.
15)
Pathological features
In a review of neonatal mortality of 50 species of wild ungulates, Kirkwood
et al. (1987) found excessively high mortality of the non-seasonal breeders,
including sitatungas and attributed this mainly to management practices.
Okoh et al. (1987) reported a fatal case of heartwater disease due to
Cowdria ruminantium in a captive sitatunga. Walker et al. (1993)
reported a new tick infection due to a novel Ixodid tick (Rhipicephalus
aquatilis) in sitatungas of Tanzania, Uganda and Zambia. In a survey
of artiodactyl species at Whipsnade, Flach et al. (2002) identified DNA
of gamma herpesviruses in several carrier species, including the Sitatunga.
A ten-year-old Sitatunga studied by Yanai et al. (2001) had calcific inclusions
underneath the tongue, identified as "calcinosis circumscripta".
Kaneene et al. (1985) studied the frequency of parasitic infection in
ruminants kept at the Detroit zoo and were discouraged by the relatively
ineffectively treated diseases in these animals.
Griner (1983) autopsied 36 sitatungas and found as main cause of death
trauma and stress-related features. Four animals died from pulmonary tuberculosis
due to Mycobacterium bovis.
16)
Physiologic data
I am not aware of any data.
17)
Other resources
A number of cell strains are kept in the "Frozen Zoo" of the
Zoological Society of San Diego (CRES) and may be obtained by contacting
Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
There is need to study the putative evolution of the tribe Tragelaphini
with modern cytogenetic study, such as FISH. There is a need for the study
of early implantation stages and endocrine features of gestation.
Acknowledgement
I appreciate very much the help of the pathologists at the San Diego Zoo.
References
Atkinson, Y.H., Gogolin-Ewens, K.J., Hounsell, E.F., Davies, M.J., Brandon,
M.R. and Seamark, R.F.: Characterization of placentation-specific binucleate
cell glycoprotein possessing a novel carbohydrate. J. Biol. Chem. 268:26679-26685,
1993.
Cotton, W.B.: The sitatunga (Tragelaphus spekii Sclater). Proc. Zool. Soc. London 143-144, 1935.
Densmore, M.A.: Reproduction of sitatunga, Tragelaphus spekei in captivity. Intern. Zoo Yearb. 20:227-229, 1980.
Flach, E.J., Reid, H., Pow, I. and Klemt, A.: Gamma herpesvirus carrier status of captive artiodactyla. Re. Vet. Sci. 73:93-99, 2002.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hassanin, A. and Douzery, E.J.: The tribal radiation of the family Bovidae (Artiodactyla) and the evolution of the mitochondrial cytochrome b gene. Mol. Phylogenet. Evol. 13:227-243, 1999.
Hradecky, P.: Uterine morphology in some antelopes. J. Zoo Anim. Med. 13:132-136, 1982.
Hradecky, P.: Placental morphology in African antelopes and giraffes. Theriogenology 20:725-734, 1983.
Hradecky, P., Benirschke, K. and Stott, G.G.: Implications of the placental structure compatibility for interspecies embryo transfer. Theriogenology 28:737-746, 1987.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Kaneene, J.B., Taylor, R.F., Sikarskie, J.G., Meyer, T.J. and Richter, N.A.: Disease patterns in the Detroit zoo: a study of mammalian populations from 1973 through 1983. J. Amer. Vet. Med. Assoc. 187:1166-1169, 1985.
Kirkwood, J.K., Gaskin, C.D. and Markham, J.: Perinatal mortality and season of birth in captive wild ungulates. Vet. Rec. 120:386-390, 1987.
Koulischer, L., Tijkens, J. and Mortelmans, J.: Mammalian Cytogenetics II. The chromosomes of a male situtunga (Tragelaphus spekii Sclater). Acta Zool. Pathol. Antv. 43:143-147, 1967.
Koulischer, L., Tijkens, J. and Mortelmans, J.: Chromosome studies of a fertile mammalian hybrid: the offspring of the cross bongo x Sitatunga (Bovoidea). Chromosoma 41:265-270, 1973.
Matthee, C.A. and Davis, S.K.: Molecular insights into the evolution of the family Bovidae: a nuclear DNA perspective. Mol. Biol. Evol. 18:1220-1230. 2001.
Matthee, C.A. and Robinson, T.J.: Cytochrome b phylogeny of the family Bovidae: Resolution within the Alcelaphini, Antilopini, Neotragini, and Tragelaphini. Mol. Phylogenet. Evol. 12:31-46, 1999.
Mentis, M.T.: A review of some life history features of the large herbivores of Africa. The Lammergeyer 16:1-89, 1972.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Okoh, A.E., Oyetunde, I.L. and Ibu, J.O.: Heartwater infection (cowdriosis) in a Sitatunga (Tragelaphus spekei) in Nigeria. J. Wildl. Dis. 23:211-214, 1987.
Petit, P., Vermeesch, J.R., Marynen, P. and De Meurichy, W.: Comparative cytogenetic study in the subfamily Tragelaphinae. Proc. 11th Europ. Coll. Cytogenet. Domest. Anim. Pp. 109-113, 1994.
Vrba, E.S. and Schaller, G.B.: Antelopes, Deer, and Relatives. Fossil Record, Behavioral Ecology, Systematics, and Conservation. Yale University Press, New Haven, 2000.
Walker, J.B., Keirans, J.E. and Pegram, R.G.: Rhipicephalus aquatilis sp. nov. (Acari: Ixodidae), a new tick species parasitic mainly on the Sitatunga, Tragelaphus spekei, in east and central Africa. Onderstepoort J. Vet. Res. 60:205-210, 1993.
Wallace, C.: Cytogenetic investigations of certain free-roaming wild animals in the Kruger Park. Ph.D. Thesis, University of Witwatersrand, Johannesburg, 1976.
Wallace, C.: Chromosomal evolution in the antelope tribe Tragelaphini. Genetica 48:75-80, 1978.
Wooding, F.B.: The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 31:31-39, 1982.
Wooding, F.B., Morgan, G. and Adam, C.L.: Structure and function in the ruminant synepitheliochorial placenta: central role of the trophoblast binucleate cell in deer. Microsc. Res. Tech. 38:88-99, 1997.
Wurster, D.H. and Benirschke, K.: Chromosome studies in the superfamily Bovoidea. Chromosoma 25:152-171, 1968.
Wurster, D.H., Benirschke, K. and Noelke, H.: Unusually large sex chromosomes in the Sitatunga (Tragelaphus spekei) and the blackbuck (Antilope cervicapra). Chromosoma 23:317-323, 1968.
Yanai, T., Noda, A., Kawakami, S., Sakai, H., Lackner, A.A. and Masegi, T.: Lingual calcinosis circumscripta in a captive sitatunga. J. Wildl. Dis. 37:813-815, 2001.