| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: March 14, 2005. |
Phodopus sungorus
Order: Rodentia
Family: Muridae [Cricetinae] (some authorities list this species under Cricetidae)
1) General Zoological Data
Dwarf hamsters from Siberia are common and they also make attractive pets. Several varieties of dwarf hamsters exist; the one described here is characterized by its extremely short tail. Piechocki (1969) summarized much of the behavior and biology of these animals and of their relatives. He also gives distribution maps. The Siberian dwarf hamster, or small desert hamster, is represented by two species, P. sungorus and the smaller P. roborovskii, according to Nowak (1999). These two species are also chromosomally distinct. Musser & Carleton (1993), however, recognize P. campbelli as an additional, separate species that was formerly grouped as subspecies under P. sungorus but was separated in 1987 by Pavlinov & Rossolimo from P. sungorus . The dwarf hamsters are described as coming from northeastern Mongolia , eastern Russia and western China (Musser & Carleton, 1993). Schmid et al. (1986) have the following to say about their distribution: “ The genus Phodopus (Rodentia, Cricetinae) comprises two species of Asiatic hamsters. P. sungorus inhabits the steppes of Ishim (Ural) to the west and up into the northern regions of Mongolia to the east. In the southeast, its habitat extends to the Balkashsea, Djungaria, and Altai. Whereas P. sungorus sungorus is a typical inhabitant of the steppes, the subspecies P. sungorus campbelli lives in the periodically dry mudflats of Mongolia . The second species, P. roborovskii, inhabits the northern and northeastern parts of the Gobi Desert , as well as the provinces of Shansi , Nan-Shang, and Zaidan (Argyropulo, 1933).
Gotch (1979) describes the origin of the name as follows: phos (Gr), genitive phodos, a burn, a blister – pous (Gr) the foot; the tubercles on the soles of the feet form a blister-like mass – sungorus is from Djungaria, a vast valley between the Altai Mountains and the Tienshan Mountains in Sinkiang, China; the name was given by Pallas in 1777. It also inhabits Siberia and Manchuria .
The animals make excellent pets as they are docile and easily tamed. Many web sites have been created by enthusiasts that are devoted to these two species; they provide extensive information on care, the various coat colors, etc. The differences in coat color that have developed gradually in pet hamsters and in different breeding groups held in captivity are there listed in these web sites and, for some varieties, their genes have been defined. The longevity is over 3 years in P. s. campbelli, a species that was named after Campbell who discovered it in 1902.
 |
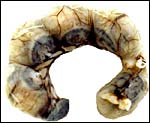
Phodopus sungorus. |
 |
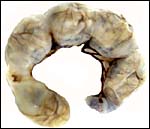
Phodopus sungorus. |
2) General Gestational Data
Birth occurs 18-19 days after breeding and litters range from one to nine animals according to Nowak (1999). Jones & Wynne-Edwards (2000) described an interesting difference in behavior of the males that differs between P. sungorus and P. campbelli during birth of the young: The latter species' males actively participate in the birthing process with removal of membranes, opening the airways of young, and licking amnionic fluid; the former species' males do not share these behaviors. Another remarkable feature is that the second and the subsequent litters have a post-implantational diapause of around 18 days (Newkirk et al., 1997).
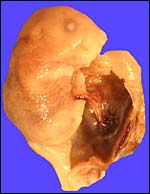
The uterus of P. sungorus in the present study was kindly donated by Dr. K.E. Wynne-Edwards, and it is from the seventeenth day of gestation . [Her laboratory differentiates between “Siberian” ( P. sungorus ) and “Djungarian” ( P. campbelli ) hamsters.] The uterus contained 4 well-developed fetuses in one horn and one normal embryo in the other horn whose other embryos were used for different purposes. At the tubal end of the horn with four fetuses there was a markedly reduced and degenerating fifth embryo. A representative normal male fetus of this gestation weighed 1.4 g after formalin fixation and measured in 2 cm crown-rump length. The entire uterus as shown in the following pictures weighed 8.6 g after formalin fixation. Gestation is normally 18 (to ?20) days long and up to 9 embryos may be present. Maternal weights vary between 20 and 50 g.
3) Implantation
Implantation in rodents has been described in great detail in my Chapter on the Mouse placenta. It is essentially similar in hamsters (Mossman, 1987) but I have had no specimens to examine of an early gestation in this species. According to data from endocrine studies, implantation occurs on day 5, after transiting to the uterus on day 3 (Erb & Wynne-Edwards, 1993) and it is anti-mesometrial.
4) General Characterization of the Placenta
The placental disk has a hemotrichorial relation to the maternal organism in Syrian hamsters (and I believe this to be true in Phodopus ). The placental disk consists is ovoid with the typical rodent labyrinth, peripheral spongiotrophoblast and giant cells. A villous inverted yolk sac membrane has its separate vascularization, and it is permanent on the anti-mesometrial side of the uterus. There is no allantoic sac. Ritschard & Hillemann (1967) followed in great detail the changing patterns of reticulin, collagen and elastin deposition in the placentas and uteri of Syrian hamsters. No such studies are known of dwarf hamsters.
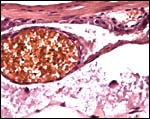
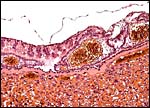
5) Details of fetal/maternal barrier
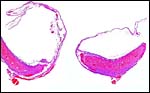
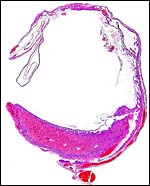
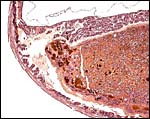
A large maternal vessel centrally perforates the labyrinth in which the larger maternal vessels border the trophoblast and fetal capillaries. The placenta is hemochorial (trichorial). There is no pigment and trophoblastic invasion into the uterus is only minimally present in this term gestation. Numerous mitoses were found in the trophoblast.
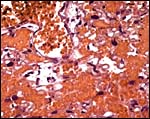 |
Higher magnification of the placental labyrinth. |
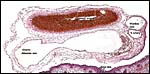
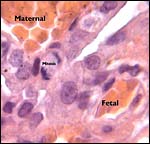 |
Hemotrichorial layer between maternal and fetal bloods. |
6) Umbilical cord
The umbilical cord is very delicate and can be seen in the next photograph. It measured 0.8 cm in length and was not spiraled. It consisted of the allantoic and vitelline portions that split before the former reaches the disk. Each portion has two blood vessels, with the vitelline vessels having practically no musculature. Remnants of the former vitelline duct have regressed by this time (17 days).
7) Uteroplacental circulation
There are no published data.
8) Extraplacental membranes
The membranes of the placental sac that surrounds the embryo consist of a very thin avascular amnion, peripheral to which is the vascularized inverted yolk sac membrane. There is no allantoic sac.
9) Trophoblast external to barrier
Syrian hamster placentas are also characterized by their extensive trophoblastic invasion of the maternal arterioles as well as the mesometrial vessels (Pijnenborg et al., 1974; Burek et al., 1979). Such infiltration was not found to any extent in the maternal blood vessels of these term gestation placental/uterine vessels. Nor did the vessels show any evidence of having been modified in the past. I do not know why this trophoblastic invasion that is so characteristic for Syrian hamsters occurs only so minimally in Phodopus .
10) Endometrium
Decidualization is readily produced in Syrian hamster uteri by histamine and by physical stimuli (review by Abrahamson & Zorn, 1993). While such changes may occur early in the gestation of dwarf hamsters, no decidualization was present in the term gestation here studied.
11) Various features
A true subplacenta as in hystricidae was not found in these implantations.
12) Endocrinology
A useful review of reproduction and endocrine findings on hamsters in general is found by Freeman & Goldman (1998). It considers primarily the adjustments of photoperiods from pineal melatonin secretion and, for Siberian hamsters it suggests that fetuses are ‘imprinted' with photoperiodic knowledge. But, clearly the most incisive endocrine and behavioral studies done in dwarf hamsters come from the laboratory of Dr. K.E. Edwards whose numerous papers are readily accessible through PubMed. Thus, the pre-implantation endocrinology was described by Erb & Wynne-Edwards (1994), and Edwards et al. (1994), as well as by McMillan & Wynne-Edwards (1998) who investigated numerous parameters of endocrine changes during gestation of P. campbelli, including their behavioral correlates. They found a surge of prolactin in early pregnancy and stable progesterone concentrations. After days 8-11 the prolactin surges abated, only to increase again in late pregnancy, and the CL began to secrete estradiol after 8 days. Later the CLs increased and progesterone concentrations doubled. Late gestation oophorectomy led to fetal loss and the placenta did not contain extractable progesterone. Roy & Wynne-Edwards (1995) followed endocrine parameters during lactation in some detail as well.
Edwards et al. (1998) described the temporal and endocrine aspects of “reproductive aging” which commences as early as at age 8 months.
13) Genetics
Phodopus sungorus has 28 distinct chromosomes, while P. roborovskii has a diploid number of 2n=34. Schmid et al. (1986) have reviewed the prior Russian publications on these hamsters and provided detailed information on the homoeologies using several banding techniques. They also defined the NOR regions and hypothesized that a number of Robertsonian fusions as well as inversions must have led to the evolution of the karyotypes. Fertile, subfertile and sterile hybrids between P. sungorus and P. campbelli have been described and descriptions of their meiotic irregularities were provided by Safronova et al. (1999). I am not aware, however, that hybrids between the two karyotypically different species have been produced. Bigger & Savage (1976) defined the NOR regions in two species of dwarf hamster, and Das & Savage (1978) described the replication pattern of Djungarian hamsters.
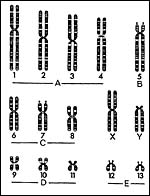 |
Idiogram of Phodophus sungorus (modified from: Bigger & Savage, 1976). |
14) Immunology
I am not aware of any published studies on the immune function of dwarf hamsters.
15) Pathological features
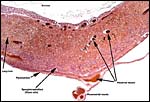
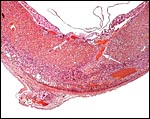
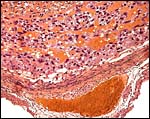
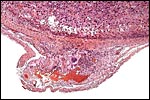
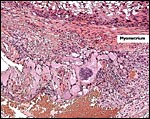
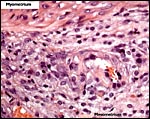
The biomedical literature does not list pathological features for this species although numerous examples for Syrian hamsters are listed, and spontaneous diabetes is described for Chinese hamsters. Kakpalova et al. (1980) produced somatic cells hybrids by fusing cells with malignant SV40-transformed fibroblasts and they derived tumors from these. Similarly, Sokova & Pogosianz (1989) induced sarcomas and adenocarcinomas by treatment with 3-methylcholanthrene, and then described the ensuing alterations of chromosomes 3p, 7q, 8q, and of the X-chromosome. The fact that the most terminal gestational sac of the uterus described here was necrotic (next photographs) indicates perhaps that the circulation of that uterine portion was deficient for the development of an additional fetus. Spontaneous chromosomal errors as causes of fetal demise have not been described.
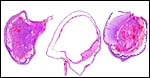 |
Cross sections through the dead embryo at the end of the uterus. |
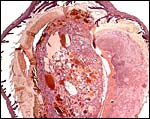 |
Dead, aborting embryo and placental disk. |
16) Physiologic data
Various scent glands have been described at the corners of eyes, behind ears and genitalia of dwarf hamsters. Because the handling of these animals so rapidly induces a significant elevation of cortisol and prolactin levels, Reburn & Wynne-Edwards (2000) showed how this can be avoided by switching air supply to exposure with isoflurane in oxygen, provided it is switched from a distance to the cages.
17) Other resources
I am not aware that cell lines or antibodies are available. Nevertheless, the animals are widely traded in pet communities and such resources could therefore be easily created.
18) Other remarks – What additional Information is needed?
Early implantations need to be studied for their possible homology to other rodents.
Acknowledgement
I am most grateful to Dr. K. E. Edwards, Canada , for providing me with this specimen. The animal photographs in this chapter also come through the courtesy of Dr. K.E. Wynne-Edwards, Canada .
References
Abrahamson, P.A. and Zorn, T.M.T.: Implantation and decidualization in rodents. J. Exp. Zool. 266 :603-628, 1993.
Argyropulo, A.J.: Die Gattungen und Arten der Hamster (Cricetinae, Murray, 1866) der Paläarktik. Z. Säugetierk. 8 :129-149, 1933.
Bigger, T.R. and Savage, J.R.: Location of nuclear organizing regions on the chromosomes of the Syrian hamster ( Mesocricetus auratus ) and the Djungarian hamster ( Phodopus sungorus ). Cytogenet. Cell Genet. 16 :495-504, 1976.
Burek, J.D., Goldberg, B., Hutchins, G. and Strandberg, J.D.: The pregnant hamster as a model to study intravascular trophoblasts and associated maternal blood vessel changes. Vet. Pathol. 16 :553-566, 1979.
Das, R.K. and Savage, J.R.: Chromosome replication patterns in the Djungarian hamster ( Phodopus sungorus ). Chromosoma 67 :165-176, 1978.
Edwards, H.E., Jenkins, K.L., Mucklow, L.C., Erb, G.E. and Wynne-Edwards, K.E.: Endocrinology of the pregnant Djungarian hamster Phodopus campbelli . J. Reprod. Fertil. 101 :1-8, 1994.
Edwards, H.E., Tweedie, C.J., Terranova, P.F., Lisk, R.D. and Wynne-Edwards, K.E.: Reproductive aging in the Djungarian hamster, Phodopus campbelli. Biol. Reprod. 58 :842-848, 1998.
Edwards, H.E., Tweedie, C.J., Terranova, P.F., Lisk, R.D. and Wynne-Edwards, K.E.: Reproductive aging in the Djungarian hamster, Phodopus campbelli . Biol. Reprod. 58 :842-848, 1998.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole , Dorset , 1979.
Erb, G.E. and Wynne-Edwards, K.E.: Preimplantation endocrinology in the Djungarian hamster ( Phodopus campbelli ): progesterone, estrogen, corpora lutea, and embryonic development. G. - Biol. Reprod. 49 :822-830, 1993.
Erb, G.E. and Wynne-Edwards, K.E.: Prolactin, follicle-stimulating hormone, and luteinizing hormone levels during preimplantation in the Djungarian hamster ( Phodopus campbelli ). Biol. Reprod. 50 :1328-1333, 1994.
Freeman, D.A. and Goldman, B.D.: Cricetidae (Hamsters and Lemmings). Pp. 739-748, in: Encyclopedia of Reproduction, E. Knobil and J.D. Neill, eds. Vol. I. Academic Press, San Diego , CA 1998.
Jones, J.S. and Wynne-Edwards, K.E.: Paternal hamsters mechanically assist the delivery, consume amniotic fluid and placenta, remove fetal membranes, and provide parental care during the birth process. Horm. Behav. 37 :116-125, 2000.
Kakpakova, E.S., Malahova, E.M., Massino, J.S. and Pogosianz, H.E.: Somatic cell hybrids of the Djungarian hamster: malignancy and evolution of the karyotype. Carcinogenesis 1 :539-545, 1980.
Newkirk, K.D., Mcmillan, H.J. and Wynne-Edwards, K.E.: Length of delay to birth of a second litter in dwarf hamsters ( Phodopus ): evidence for post-implantation embryonic diapause. J. Exp. Zool. 278 :106-114, 1997.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
McMillan, H.J. and Wynne-Edwards, K.E.: Evolutionary change in the endocrinology of behavioral receptivity: Divergent roles for progesterone and prolactin within the genus Phodopus. Biol. Reprod. 59 :30-38, 1998.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Musser, G.G. and Carleton, M.D.: Muridae, pp. 501-755, in: Wilson , D.E. and Reeder, D.A.M., ed.: Mammal Species of the World. A Taxonomic and Geographic Reference. 2 nd ed. Smithsonian Institution Press, Washington , DC , 1993.
Newkirk, K.D., Mcmillan, H.J. and Wynne-Edwards, K.E.: Length of delay to birth of a second litter in dwarf hamsters ( Phodopus ): evidence for post-implantation embryonic diapause. J. Exp. Zool. 278 :106-114, 1997.
Piechocki, R.: Die Mäuseverwandten. Pp. 301-399, in Grzimeks Tierleben. Vol. XI. Kindler-Verlag, Zürich, 1969.
Pijnenborg, R., Robertson, W.B. and Brosens, I. : The arterial migration of trophoblast in the uterus of the golden hamster, Mesocricetus auratus . J. Reprod. Fertil. 40 :269-280, 1974.
Reburn, C.J. and Wynne-Edwards, K.E.: Cortisol and prolactin concentrations during repeated blood sample collection from freely moving, mouse-sized mammals ( Phodopus spp.) Comp. Med. 50 :184-198, 2000.
Ritschard, R.L. and Hillemann, H.H.: The patterns of development and distribution of collagen, reticulin, and elastin in the placentae of the golden hamster ( Mesocricetus aureus Waterhouse). Trans. Amer. Microsc. Soc. 86 :294-303, 1967.
Roy , B.N. and Wynne-Edwards, K.E.: Progesterone, estradiol, and prolactin involvement in lactation, including lactation following a postpartum mating, in the Djungarian hamster ( Phodopus campbelli ). Biol. Reprod. 52 :855-863, 19995.
Safronova, L.D., Cherepanova, E.V. and Vasil'eva, NIu.: Characteristics of the first meiotic division in hamster hybrids obtained by backcrossing Phodopus sungorus and Phodopus campbelli . Genetika 35 :237-242, 1999 (in Russian).
Schmid, M., Haaf, T., Weis, H. and Schempp, W.: Chromosomal homoeologies in hamster species of the genus Phodopus (Rodentia, Cricetinae). Cytogenet. Cell Genet. 43 :168-173, 1986.
Sokova, O.I. and Pogosianz, H.E.: Non-random karyotypic changes in Djungarian hamster tumors induced by 3-methylcholanthrene. Arch. Geschwulstforsch. 59 :399-406, 1989.