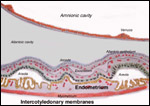
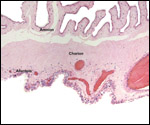
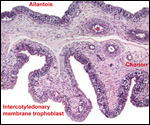
| |
Numerous
infectious diseases affect sheep. They have been summarized in the veterinary
literature, e.g. by Smith et al. (1972). Among these is brucellosis due
to infection with Brucella ovis. It causes mainly epididymitis in
rams but may affect the female generative tract and lead to abortion with
placental inflammation.
16)
Physiological data
The sheep has been used for numerous physiological studies; many are designed
to better understand human prenatal development. Thus, Barron (1951) reviewed
in some detail the transfer of oxygen. He and others have studied this
transfer at high altitude and compared it with data found at sea level.
Liggins (1969) reviewed the endocrine signals for the onset of labor and
parturition. Kleemann et al. (2001) showed that progesterone administration
in very early pregnancy enhances fetal growth. Alexander (1964) removed
up to 84 caruncles from Merino sheep prior to mating in order to assess
the impact on placentation. While fertility was not affected, the ewes
in which sixty or more caruncles had been removed delivered prematurely
and the lambs had reduced birth weights. The caruncle removal was compensated
by an increased size of the cotyledons. Worthington et al. (1981) carried
out similar reductions with electrocautery. They found a similar effect
on fetal growth. Creasy et al. (1972) embolized fetal cotyledons with
microspheres between days 96 and 125 and found significant reduction in
fetal size. The affected cotyledons showed hyalinization and necrosis
with marked reduction in size. This was followed by similar experiments
of Duncan et al. (2000). These investigators examined the consequences
of interference with placentation on fetal brain development. They identified
significant damage to the white matter of the CNS in the growth-restricted
lambs. There are many other, similar studies that probe fetal CNS development
by restricting placental performance. For instance, Dawes & Mott (1964)
altered oxygen supply to fetal lambs and studied their responses. Keunen
et al. (1997) found no CNS damage with cord occlusion of up to ten minutes,
but Ball et al. (1994) found that seizures took place when there was severe
cord occlusion for 90 minutes. Ikeda et al. (1998) sought to explain the
CNS lesions after cord occlusion by brain lipid peroxidation. Mallard
et al. (1992) found primarily hippocampal lesions after occlusion, while
Clapp et al. (1988) found mostly white matter damage after intermittent
occlusion of the cord's vessels. Gardner et al. (2002) used sheep placentation
to study the effect of periodic cord occlusion. They found that the number
of caruncles did not decrease, but their weights decreased and appearances
changed.
Gilbert et al. (1996) showed that technetium crossed the ovine placenta
more rapidly than the smaller sodium ion. Ovine urine production was studied
by Ross, et al. (1988) and by many other investigators who are listed
by these authors. Ross et al. found in this carefully executed protocol
(days after surgical canulations, standing ewes, 4-hour experiment, careful
electrolyte measurements) that the amount of allantoic fluid was greater
than that of amniotic fluid; that fetal urination is greater into the
allantoic sac (through urachus) than the amnionic sac (through urethra);
that transmembranous flow probably exists; that lung fluid participates
in amnionic fluid composition and swallowing in disposing of it. These
are difficult experiments whose interpretation is complicated by the presence
of two large fluid-filled sacs in the ovine placenta. They are not directly
transferable to human amnionic fluid metabolism for that reason. With
Barron we catheterized the urachus in an instrumented sheep that was suspended
in a water bath. We found that urine production decreased markedly over
a few hours when all urine from the urachus was collected externally.
Its osmolality also increased and much fructose was contained in the urine.
Urination increased rapidly when water was injected into the allantoic
cavity. We conjectured that the water was rapidly absorbed by the allantoic
circulation and rehydrated the water-deprived fetus. Matsumoto et al.
(2000) ligated the fetal esophagus and urachus and were unable to produce
polyhydramnios, as had been their aim. This suggested to them that transmembranous
transport must be large.
More recently, Daneshmand et al. (2003) investigated the regulation of amnionic fluid volume in sheep and suggested that vascular endothelial growth factor increases intramembranous absorption by microvesicular transport.
17)
Other resources
Cell strains of various sheep species are available from CRES at the Zoological
Society of San Diego.
18) Other remarks - What additional Information is needed?
Relatively few noninfectious placental lesions have been described in
sheep. More knowledge is also needed on umbilical cords. Mossman (1987)
pointed out that more information is needed on the insertion of the cords
of multiple gestations. Because there is still some uncertainty as to
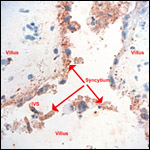
the true origin of the syncytial giant cells, additional studies are warranted.
Acknowledgement
Most of the animal photographs in these chapters come from the Zoological
Society of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Alexander, G.: Studies of the placenta of the sheep (Ovis aries L.).
Placental size. J. Reprod. Fertil. 7:289-305, 1964.
Alexander,
G.: Studies on the placenta of the sheep (L.). Effect of surgical reduction
in the number of caruncles. J. Reprod. Fertil. 7:307-322, 1964.
Arvy,
L. and Pilleri, G. The Cordon Ombilical (Funis umbilicalis). Verlag Hirnanatom.
Instit., Ostermundigen, Switzerland, 1976.
Assheton,
R.: VI. The morphology of the ungulate placenta, particularly the development
of that organ in the sheep, and notes upon the placenta of the elephant
and hyrax. Phil. Trans. Roy. Soc. London, Series B 198:143-220, 1906.
Ball,
R.H., Parer, J.T., Caldwell, L.E. and Johnson, J.: Regional blood flow
and metabolism in ovine fetuses during severe cord occlusion. Amer. J.
Obstet. Gynecol. 171:1549-1555, 1994.
Barron,
D.H.: Some aspects of the transfer of oxygen across the syndesmochorial
placenta of the sheep. The Yale J. Biol. Med. 24:169-190, 1951.
Bautzmann,
H. and Schroder, R.: Vergleichende Studien uber Bau und Funktion des Amnions.
Das Amnion der Säuger am Beispiel des Schafes (Ovis aries).
Z. Zellforsch. 43:48-63, 1955.
Bourne,
G.L.: The Human Amnion and Chorion. Lloyd-Luke, London, 1962.
Bunch,
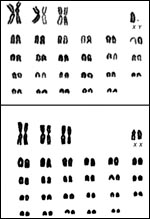
T.D., Foote, W.C. and Spillett, J.J.: Sheep-goat hybrid karyotypes. Theriogenology
6:379-385, 1976.
Clapp,
J.F., Peress, N.S., Wesley, M. and Mann, L.I.: Brain damage after intermittent
partial cord occlusion in the chronically instrumented fetal lamb. Amer.
J. Obstet. Gynecol. 159:504-509, 1988.
Creasy,
R.K., Barrett, C.T., Swiet, M.de, Kahanpää, K.V. and Rudolph,
A.M.: Experimental intrauterine growth retardation in the sheep. Amer.
J. Obstet. Gynecol. 112:566-573, 1972.
Daneshmand, S.S., Cheung, C.Y. and Brace, R.A.: Regulation of amniotic fluid volume by intramembranous absortion in sheep: Role of passive permeability and vascular endothelial growth factor. Amer. J. Obstet. Gynecol. 188:786-793, 2003.
Davies,
J.: Correlated anatomical and histochemical studies on the mesonephros
and placenta of the sheep. Amer. J. Anat. 91:263-299, 1952.
Davies,
J. and Wimsatt, W.A.: Observations on the fine structure of the sheep
placenta. Acta anat. 65:182-223, 1966.
Dawes,
G.S. and Mott, J.C.: Changes in O2 distribution and consumption in foetal
lambs with variation in umbilical blood flow. J. Physiol. 170:524-540,
1964.
Dent,
J., McGovern, P.T. and Hancock, J.L.: Immunological implications of ultrastructural
studies of goat x sheep hybrid placentae. Nature 231:115-117, 1971.
Duncan,
J.R., Cock, M.L., Harding, R. and Rees, S.M.: Relation between damage
to the placenta and the fetal brain after late-gestation placental embolization
and fetal growth restriction in sheep. Amer. J. Obstet. Gynecol. 183:1013-1022,
2000.
Gardner , D.S., Ward, J.W., Giussani, D.A. and Fowden, A.L.: The effect of a reversible period of adverse intrauterine conditions during late gestation on fetal and placental weight and placentome distribution in sheep. Placenta 23:459-466, 2002.
Gardner, D.S., Ward, J.W., Giussani, D.A. and Fowden, A.L.: The effect
of a reversible period of adverse intrauterine conditions during late
gestation on fetal and placental weight and placentome distribution in
sheep. Placenta 23:459-466, 2002.
Gilbert,
W.M., Newman, P.S., Eby-Wilkens, E. and Brace, R.A.: Technetium Tc 99m
rapidly crosses the ovine placenta and intramembranous pathway. Amer.
J. Obstet. Gynecol. 175:1557-1562, 1996.
Gray,
A.P.: Mammalian Hybrids. Second edition. A Check-List with Bibliography.
Commonwealth Agricultural Bureaux, Farnham Royal, Slough, UK, 1972.
Houston, F., Foster, J.D., Chong, A., Hunter, N. and Bostock, C.J.: Transmission
of BSE by blood transfusion in sheep. Lancet 356:999-1000 (and 955-956
for Comments), 2000.
Hyde, S.R. and Benirschke, K.: Gestational psittacosis: Case report and
literature review. Modern Pathol. 10:602-607, 1997
Ikeda, T., Murata, Y., Quilligan, E.J., Parer, J.T., Doi, S. and Park,
S-D.: Brain lipid peroxidation and antioxidant levels in fetal lambs 72
hours after asphyxia by partial umbilical cord occlusion. Amer. J. Obstet.
Gynecol. 178:474-478, 1998.
Johnson, G.A., Burghardt, R.C., Joyce, M.M., Spencer, T.E., Bazer, F.W., Pfarrer, C. and Gray , C.A. : Osteopontin expression in uterine stroma indicates a decidualization-like differentiation during ovine pregnancy. Biol. Reprod. 68:1951-1958, 2003.
Keisler, D.H.: Sheep and Goats. In, Encyclopedia of Reproduction. E. Knobil
and J.D. Neill, eds. Vol. 4, pp. 479-492. Academic Press, San Diego, 1999.
Keunen, H., Blanco, C.E., Reempts, J.L.H.van and Hasaart, T.H.M.: Absence
of neuronal damage after umbilical cord occlusion of 10, 15, and 20 minutes
in midgestation fetal sheep. Amer. J. Obstet. Gynecol. 176:515-520, 1997.
King, G.J., Atkinson, B.A. and Robertson, H.A.: Implantation and early
placentation in domestic ungulates. J. Reprod. Fertil. Suppl. 31:17-30,
1982.
Kleemann, D.O., Walker, S.K., Hartwich, K.M., Fong, L., Seamark, R.F.,
Robinson, J.S. and Owens, J.A.: Fetoplacental growth in sheep administered
progesterone during the first three days of pregnancy. Placenta 22:14-23,
2001.
Lawn, A.M., Chiquoine, A.D. and Amoroso, E.C.: The development of the
placenta in the sheep and goat: An electron microscopic study. J. Anat.
105:557-578, 1969.
Lee, C.S., Gogolin-Ewens, K. and Brandon, M.R.: Comparative studies on
the distribution of binucleate cells in the placentae of deer and cow,
using monoclonal antibody, SBU-3. J. Anat. 147:163-179, 1986.
Liggins, G.C.: The foetal role in the initiation of parturition in the
ewe. CIBA Fndt. Symp. On Foetal Anatomy. G.E.W. Wolstenholm & M. O'Connor,
eds. Pp. 218-231, 1969.
Long, S.E. and Williams, C.V.: Frequency of chromosomal abnormalities
in early embryos of the domestic sheep (Ovis aries). J. Reprod.
Fertil. 58:197-201, 1980.
Ludwig, K.S.: Zur Feinstruktur der materno-fetalen Verbindung im Placentom
des Schafes (Ovis aries L.). Experientia 18:212-213, 1962.
Ludwig, K.S.: Zur vergleichenden Histologie des Allantochorion. Rev. Suisse
Zool. 75:819-831, 1968.
Makowski, E.L.: Maternal and fetal vascular nets in placentas of sheep
and goats. Amer. J. Obstet. Gynecol. 100:283-288, 1968.
Mallard, E.C., Gunn, A.J., Williams, C.E., Johnston, B.M. and Gluckman,
P.D.: Transient umbilical cord occlusion causes hippocampal damage in
the fetal sheep. Amer. J. Obstet. Gynecol. 167:1423-1430, 1992.
Matsumoto, L.C., Cheung, C.Y. and Brace, R.A.: Effect of esophageal ligation
on amniotic fluid volume and urinary flow rate in fetal sheep. Amer. J.
Obstet. Gynecol. 182:699-705, 2000.
Miyazaki, H., Imai, M., Hirayama, T., Saburi, S., Tanaka, M., Maruyama,
M., Matsuo, G., Meguro, H., Nishibashi, K., Inoue, F., Djiane, J., Gertler, A., Tachi,
S., Imakawa, K. and Tachi, C.: Establishment of feeder-independent cloned caprine trophoblast
cell line which expresses placental lactogen and interferon tau. Placenta
23:613-630, 2002.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Naaktgeboren, C. and Zwillenberg, H.H.L.: Untersuchungen uber die Auswuchse
am Amnion und an der Nabelschnur bei Walen und Huftieren, mit besonderer
Berucksichtigung des europäischen Hausrindes. Acta morphol. Neerlando-Scand.
4:31-60, 1961.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins
Press, Baltimore, 1999.
Osgerby, J.C., Gadd, T.S. and Wathes , D.C. : The effects of maternal nutrition and body condition on placental and foetal growth in the ewe. Placenta 24:236-247, 2003.
Puschmann, W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher
Landwirtschaftsverlag Berlin, 1989.
Reynolds, S.R.M.: The proportion of Wharton's jelly in the umbilical cord
in relation to distention of the umbilical arteries and vein, with observations
on the folds of Hoboken. Anat. Rec. 119:365-377, 1952.
Ross, M.G., Ervin, M.G., Rappaport, V.J., Youssef, A., Leake, R.D. and
Fisher, D.A.: Ovine fetal urine contribution to amniotic and allantoic
compartments. Biol. Neonat. 53:98-104, 1988.
Silverstein, A.M., Prendergast, R.A. and Kraner, K.L.: Homograft rejection
in the fetal lamb: The role of circulating antibody. Science 142:1172-1173,
1963.
Silverstein, A.M., Thorbecke, G.J., Kraner, K.L. and Lukes, R.J.: Fetal
response to antigenic stimulus. III. ?-globulin production in normal and
stimulated lambs. J. Immunol. 91:384-395, 1963.
Smith, H.A., Jones, T.C. and Hunt, R.D.: Veterinary Pathology. Lea & Febiger, Philadelphia, 1972.
Ward, J.W., Wooding, F.B.P. and Fowden, A.L.: The effect of cortisol on
the binucleate cell population in the ovine placenta during late gestation.
Placenta 23:451-458, 2002.
Wilsmore,
A.J., Izzard, K.A., Wilsmore, B.C. and Dagnall, G.I.R.: Breeding performance
of sheep infected with Chlamydia psittaci (ovis) during their preceding
pregnancy. Vet. Rec. 130:68-70, 1990.
Wimsatt,
W.A.: New histological observations on the placenta of the sheep. Amer.
J. Anat. 87:391-458, 1950.
Wooding,
F.B.P.: Role of binucleate cells in fetomaternal cell fusion at implantation
in the sheep. Amer. J. Anat. 170:233-250, 1950.
Wooding,
F.B.P.: Frequency and localization of binuclear cells in the placentome
of ruminants. Placenta 4:527-540, 1983.
Wooding,
F.B.P., Flint, A.P.F., Heap, R.B. and Hobbs, T.: Autoradiographic evidence
for migration and fusion of cells in the sheep placenta: Resolution of
a problem in placenta classification. Cell Biol. Internat. Reports 5:821-827,
1981. See also: Cell Biol. Int. Rep. 5:821-827, 1981. See also: Cell Biol. Int. Rep. 5:821-827, 1981.
Wooding, F.B., Flint , A.P., Heap, R.B., Morgan, G., Buttle, H.L. and Young, I.R.: Control of binucleate cell migration in the placenta of sheep and goat. J. Reprod. Fertil. 76:499-512, 1986.
Wooding,
F.B., Flint, A.P., Heap, R.B., Morgan, G., Buttle, H.L. and Young, I.R.:
Control of binucleate cell migration in the placenta of sheep and goat.
J. Reprod. Fertil. 76:499-512, 1986.
Worthington,
D., Piercy, W.N. and Smith, B.T.: Effects of reduction of placental size
in sheep. Obstet. Gynecol. 58:215-221, 1981. |