| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last
updated: August 5, 2005. |
Enhydra lutris
Order: Carnivora
Family: Mustelidae
1) General Zoological Data
This single species was once widely distributed over the Pacific Ocean but its numbers have been severely reduced in recent years. This reduction of the sea otter population, largely because of the impact of the fur trade, has also led to a significant reduction in their genetic diversity (Larson et al., 2002). Nowak (1999) referred to the recent genetic conformation that three subspecies have existed and considered this understanding to be important for reintroduction purposes.
The name comes from: "enhydra" - Greek for otter, and "lutra" - Latin for otter (Gotch, 1979). A few aquaria keep and breed this species that is now protected and that has been gradually increasing again in numbers. Oil spills and competition from fishing activities pose threats, as do environmental toxins. The skin of sea otters is said to be extremely valuable and that fact has led to massive hunting in the past. The sea otter has extraordinarily thick hair which traps air for protection against the cold, as there is little subcutaneous fat for insulation. The diet of sea otters consists mostly of fish and sea urchins, and the usage of stone to crack open preyed abalone whilst floating on its back is well known and loved by the public. The animals are adored, in part for that reason.
Male sea otters weigh 22-45 kg, while females are 15-32 kg according to Estes (1980). The maximum lifespan for female sea otters is 23 years according to Nowak (1999). Thenius & Hofer (1960) considered that the sea otter is the most specialized of otters, largely because of their dentition and the structure of the pelvic bones. They related it to an earlier form of ocean-living otters.
 |
Sea otter with pup (Courtesy Bryant Austin, AGPA) |
 |
Sea otter (Courtesy Bryant Austin, AGPA) |
 |
Sea otter (Courtesy Bryant Austin, AGPA) |
Nowak (1999) states that 2% of births are multiple but that the female can successfully raise only one pup. Newborns weigh 1.4-2.3 kg and the estimated length of gestation is 4-5.5 months with some delayed implantation being likely. Antrim & Cornell (1980) summarized the poor reproductive successes of sea otters in captivity. Few animals have survived to maturity when they were raised in captivity. This is contrary to the reproductive rate of increase (15-18%) in the undisturbed wild (Estes et al., 1996). Antrim & Cornell (1980) also commented on the frequency of stillbirths and abortions in first pregnancies and suggested that the preimplantation stage may last 7-8 months, with the implantation stage being 4½-5 months. Jameson & Johnson (1993) believe that the preimplantation period is only 2-3 months long. Larson et al. (2003) indicated that the implanted gestation lasts on average 217 days. It is still unknown, however, whether the ovulation is induced.
3) Implantation
As noted earlier, there is an apparently long period of preimplantation
that may last longer than the actual gestation. But early stages of implantation
have not been witnessed, nor is the state of the blastocyst known prior
to implantation.
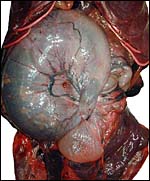
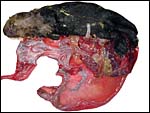
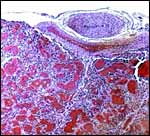
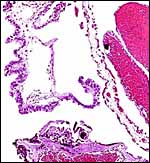
This is a labyrinthine, endotheliochorial placenta, quite similar to that of the clawless otter whose chapter should be consulted as that placenta was slightly better preserved. This specimen was made available to me through the cooperation of Melissa Miller and Sharon Toy-Choutka of the Marine Wildlife Veterinary Care and Research Center in Santa Cruz and Davis, California. It comes from a near-term pregnant female that died after being injured and strangled by a fishing line at Monterey, California. The pregnant dam weighed 17.3 kg and had a single male fetus near term that weighed 950 g after autopsy and formalin fixation. It was estimated to be 10 days premature as judged by experienced observers and had some meconium particles over its skin. The ring-shaped placenta weighed 250 g and had implanted in the left horn.
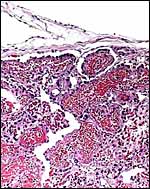
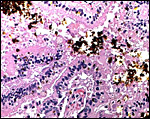
This is a lamellar, labyrinthine, endotheliochorial organ similar to that of the clawless otter (see that chapter). The difficult-to-see villi are covered by a single layer of cuboidal trophoblast without any major different cell types being discernible. Amoroso (1961) referred to the trophoblast as "syntrophoblast", but I am not certain that there is truly fusion of trophoblast, and no fine structural detail is available. The villi are composed of very delicate fibrous tissue with distended capillaries. In several sections made from this placenta, only a small hemophagous region was found at the edge of the placenta. It had crystalline pigment in- and outside of trophoblastic cells. As is the usual case with the pigment of the hemophagous organ, this also was negative with Prussian blue stains and was thus not hemosiderin. The box-shaped nature of the yellow crystals suggests hematoidin. In addition though, a saccular structure was present on the fetal surface that has several lobes and contained blood remnants (see above picture). It originates in a central region of modified columnar trophoblast shown next and diagrammed expertly as Fig. 32.1 by Mossman (1987). This lobulated structure was well described and commented upon by Sinha & Mossman (1966) and also in Burton's (1982) review of transplacental iron absorption. It was typically located in the extraembryonic space and also shown to compress the amnionic sac.
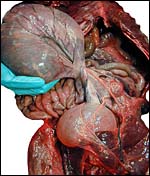
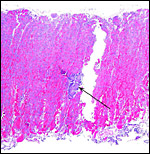
As can be seen from the gross photograph, the umbilical cord is extremely short. It has no spirals and the surface is smooth without caruncles. In addition to the three large allantoic vessels, many small blood vessels are present in the umbilical cord, as will be seen in the next photograph. The surface is composed of a single layer of amnion. A typical allantoic duct is present that opens into the complex allantoic sac.
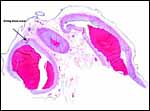 |
Incompletely sectioned umbilical cord with small allantoic vessels (one is exiting at arrow) and complex allantoic membrane ramifications at the site of ductal opening. |
There are no published studies.
8) Extraplacental membranes
The amnion is avascular and very thin it is composed of a minor amount of connective tissue and has a thin flat surface epithelium. The allantoic sac has a more columnar epithelium and contains finely distributed small blood vessels. The yolk sac, earlier to be found within the umbilical cord, has disappeared at this stage of development.
No infiltration of trophoblast into the maternal tissue was seen.
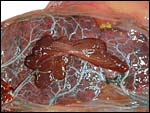
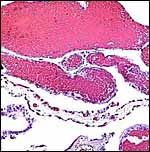
10) Endometrium
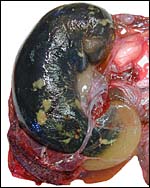
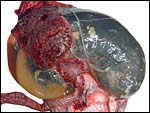
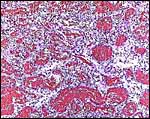
The uterus of the sea otter has been described as being long and tubular and specially adapted for bearing one or two young (Mossman, 1987). The emptied uterus weighed 170 g after fixation. A girdle-shaped region where the placenta had been attached and which was covered with debris was seen macroscopically. The remainder of the endometrium shows no decidualization and no trophoblastic infiltration was evident.
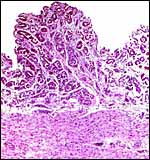 |
Endometrium from the region adjacent to the girdle-shaped implantation site. |
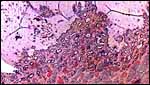 |
This is the girdle-shaped remnant of the placental attachment on the endometrium after removal of the placenta. |
Neither subplacenta nor metrial glands exist.
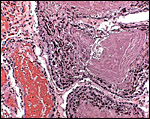
12) Endocrinology
Larson et al. (2003) validated estrogen and progesterone values of various reproductive states by fecal metabolite monitoring in hopes of improving captive reproductive management. Primary steroid disposition is via feces in sea otters. Females cycled 3-4 times a year with elevation of total estrogens and usually (but not always) followed by rise in progestagen. During the preimplantation stage there was marked elevation of progestagen. There was a dramatic increase in progestagen levels after implantation and thus pregnancy can be reliably diagnosed. A significant fall in total estrogens occurred shortly before parturition.
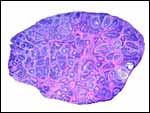 |
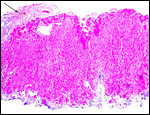
Section of one ovary to show the extensive follicular luteinization. |
Sea otters have 38 chromosomes, the majority being metacentrics (Hsu & Benirschke, 1971). The smallest acrocentric chromosome has the typical "carnivore constriction". Duffield et al. (1996) studied one male and three female animals of a breeding colony and found the male to have several banding abnormalities. They speculated that these may have a causal relation to some congenital defects seen in offspring that were not studied. Hybrids have not been described.
 |
Karyotypes of male and female sea otters (from Hsu & Benirschke, 1971). |
King et al. (1996) have cloned and studied the sequence of the IL-6 cDNA fragments of seal, killer whale and sea otter. They found it conserved and the Il-6 of sea otter and seal were closely related to other carnivores, while the IL-6 of killer whale was more similar to artiodactyla.
15)
Pathological features
Injuries from fishing activities and from the entangling in fishing lines
with subsequent infection are common. It has also been suspected that
the animals may be affected, as are sea lions and perhaps birds, by the
ingestion of domoic acid (Gulland, 2000) [Neural symptoms, placental necrosis].
This acid is produced by the plumes of a specific plankton, Pseudo-nitzschia
australis, and studies are underway to further define this. When
crude oil spills led to the deaths of sea otters, various lesions were
correlated with these fatalities (Lipscomb et al., 1993). These included
emphysema and lipid infiltration in liver and kidney, as well as focal
liver necrosis.
Several tumors have been reported to have occurred in this species. Kim
et al. (2002) described a lymphosarcoma in a 4 year old male. Reimer &
Lipscomb (1998) found a malignant seminoma in a stranded Alaskan sea otter
which also had herpetic inclusions and acanthocephalan helminths in the
intestines. Williams & Pulley (1981) had two female otters with leiomyomas,
while Stetzer et al. (1981) found leiomyoma, cholangiocarcinoma and pheochromocytoma
in a sea otter.
A variety of parasites have been identified in sea otters; thus, several
species of sarcocystis were reported by Dubey et al. (2001, 2003a, b).
Antibodies against Toxoplasma gondii (as well as other agents)
were identified in healthy sea otters as well, and also in several other
marine mammals (Dubey et al., 2003a). The actual toxoplasmosis with encephalitis
(and significant cardiac disease) was identified and is described in the
larger survey by Kreuder et al. (2003). Perhaps contamination from cats
etc. is able to transmit the disease
(Miller et al., 2002; 2004).
Remarkably, a case of disseminated
coccidioidomycosis occurred in an emaciated otter (Cornell et al., 1979).
Staveley et al. (2003) obtained pure cultures of a lethal strain of Bordotella
bronchiseptica from lung, abdomen and intestines of a free-ranging
animal.
There are numerous web sites on sea otters that can be accessed readily by searching in Google under "sea otter". Many contain valuable information. One of the remarkable aspects of sea otters is their skin. Nowak (1999) indicated that this species has the thickest skin, albeit without subcutaneous fat, and that it has extremely dense hair follicles (100,000/sqcm).
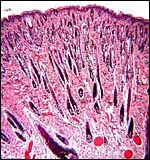 |
This piece of abdominal skin from the neonate is to show the very dense population of hair follicles in sea otters. |
Regrettably no cell lines are available to my knowledge.
18) Other remarks - What additional
Information is needed?
The length of preimplantation in this species is unknown as yet and this
may vary considerably. Nothing has been published on the preimplantation
stages, or on early implantation.
Acknowledgement
This specimen was made available to me through the cooperation of Melissa
Miller and Sharon Toy-Choutka of the Marine Wildlife Veterinary Care and
Research Center in Santa Cruz and Davis, California whose cooperation
is greatly appreciated. The photographs of the animals were kindly supplied
by Bryant Austin AGPA, Marine Wildlife Veterinary Care and Service Center,
1451 Shaffer Road, Santa Cruz, CA 95060.
References
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall's Physiology
of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown & Co. Boston, 1961.
Antrim, J.E. and Cornell, L.H.: Reproduction of the Sea otter Enhydra lutris in captivity. Intern. Zoo Yearb. 20:76-80, 1980.
Burton, G.J.: Review article. Placental uptake of maternal erythrocytes: a comparative study. Placenta 3: 407-434, 1982.
Cornell, L.H., Osborn, K.G., Antrim, J.E. Jr. and Simpson, J.G.: Coccidioidomycosis in a California sea otter (Enhydra lutris). J. Wildl. Dis. 15:373-378, 1979.
Dubey, J.R., Rosypal, A.C., Rosenthal, B.M., Thomas, N.J., Lindsay, D.S., Stanek, J.F., Reed, S.M. and Saville, W.J.: Sarcocystis neuronal infections in sea otter (Enhydra lutris): evidence for natural infections with sarcocysts and transmission of infection to opossums (Didelphis virginiana). J. Parasitol. 87:1387-1393, 2001.
Dubey, J.P., Zarnke, R., Thomas, N.J., Wong, S.K., van Bonn, W., Briggs, M., Davis, J.W., Ewing, R., Mense, M., Kwok, O.C., Romand, S. and Thulliez, P.: Toxoplasma gondii, Neospora canium, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet. Parasitol. 116:275-296, 2003a.
Dubey, J.P., Lindsay, D.S., Rosenthal, B.M. and Thomas, N.J.: Sarcocysts of an unidentified species of Sarcocystis in the sea otter (Enhydra lutris). J. Parasitol. 89:397-399, 2003b.
Duffield, D.A., Chamberlin-Lea, J. and Antrim, J.E.: A complex chromosome rearrangement in the karyotype of a wild-caught male sea otter. Endangered Species Update (in The Otter Project) 13 (#12):1-8, 1996.
Estes, J.A.: Enhydra lutris. Mammalian Species. Amer. Soc. Mammal. # 133, 1980.
Estes, J.A., Doak, D.F., Bodkin, J.R., Jameson, R.J., Monson, D., Watt, J. and Tinker, M.T. Comparative demography of sea otter populations. Endangered Species Update 13:11-13, 1996.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gulland, F.: Domoic acid toxicity in California sea lions (Zalophus californianus) stranded along the central California coast, May-October 1998. Report to the National Marine Fisheries Service Working Group on unusual Marine Mammal Mortality Events. US Dept. Commerce NOAA Technical Memorandum NMFS-OPR-17, December 2000.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 6: Folio 285, 1971. Springer-Verlag, N.Y.
Jameson, R.J. and Johnson, A.M.: Reproductive characteristics of female sea otters. Marine Mammal Science 9:156-167, 1993.
Kim, J.H., Kim, B.H., Kim, J.H., Yoo, M.J. and Kim, D.Y.: Lymphosarcoma in a sea otter (Enhydra lutris). J. Wildl. Dis. 38:616-617, 2002.
King, D.P., Schrenzel, M.D., McKnight, M.L., Reidarson, T.H., Hanni, K.D., Stott, J.L. and Ferrick, D.A.: Molecular cloning and sequencing of interleukin 6 cDNA fragments from the harbor seal (Phoca vitulina), killer whale (Orcinus orca), and Southern sea otter (Enhydra lutris nereis). Immunogenetics 43:190-195, 1996.
Kreuder, C., Miller, M.A., Jessup, D.A., Lowenstine, L.J., Harris, M.D., Ames, J.A., Carpenter, T.E., Conrad, P.A. and Mazet, J.A.: Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998-2001. J. Wildl. Dis. 39:495-509, 2003.
Larson, S., Jameson, R., Etnier, M., Fleming, M. and Bentzen, P.: Loss of genetic diversity in sea otters (Enhydra lutris) associated with the fur trade of the 18th and 19th centuries. Mol. Ecol. 11:1899-1903, 2002.
Larson, S., Casson, C.J. and Wasser, S.: Noninvasive reproductive steroid hormone estimates from fecal samples of captive female sea otters (Enhydra lutris). Gen. Comp. Endocrinol. 134:18-25, 2003.
Lipscomb, T.P., Harris, R.K., Moeller,
R.B., Pletcher, J.M., Haebler, R.J. and Ballachey, B.E.: Histopathologic
lesions in sea otters exposed to crud oil. Vet. Pathol. 30:1-11, 1993.
Miller, M.A., Gardner , I.A., Kreuder, C., Paradies, D.M., Worcester , K.R., Jessup, D.A., Dood, E., Harris, M.D., Ames, J.A., Packham, A.E. and Conrad, P.A.: Costal water runoffs is a risk factor for Toxoplasma gondii infection of southern sea otters ( Enhydra lutris nereis ). Int. J. Parasitol. 32:997-1006, 2002.
Miller, M.A., Grigg , M.E. , Kreuder, C., James, E.R., Melli, A.C., Crosbie, P.R., Jessup, D.A., Boothroyd, J.C., Brownstein, D. and Conrad, P.A.: An unusual type of Toxoplasma gondii is common in California sea otters ( Enhydra lutris nereis ) and is a cause of mortality. Int. J. Parasitol. 34:275-284, 2004.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Reimer, D.C. and Lipscomb, T.P.: Malignant seminoma with metastasis and Herpesvirus infection in a free-living sea otter (Enhydra lutris). J. Zoo Wildl. Med. 29:35-39, 1998.
Sinha, A.A. and Mossman, H.W.: Placentation of the sea otter. Amer. J. Anat. 119:521-554, 1966.
Staveley, C.M., Register, K.B., Miller, M.A., Brockmeier, S.L., Jessup, D.A. and Jang, S.: Molecular and antigenic characterization of Bordotella bronchiseptica isolated from a wild southern sea otter (Enhydra lutris nereis) with severe suppurative bronchopneumonia. J. Vet. Diagn. Invest. 15:570-574, 2003.
Stetzer, E., Williams, T.D. and Nightingale, J.W.: Cholangiocellular adenocarcinoma, leiomyoma, and pheochromocytoma in a sea otter. J. Amer. Vet. Med. Assoc. 179:1283-1284, 1981.
Thenius, E. and Hofer, H.: Stammesgeschichte der Säugetiere. Springer-Verlag, Berlin, 1960.
Williams,
T.D. and Pulley, L.T.: Leiomyomas in two sea otters, Enhydra lutris.
J. Wildl. Dis. 17:401-404, 1981.