| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: June 17, 2004. |
Callorhinus ursinus; Zalophus californianus; Arctocephalus; Phoca vitulina
Order – Pinnipedia
Family – Otariidae & Phocidae
1) General Zoological Data
Pinnipedia have generally been separated as a distinct Order although previous grouping under Carnivora has been debated (Nowak, 1999). Thus, most recently, Wozencraft (1993) gave reasons to place these animals again under Carnivora as a suborder Caniformia, and their placentation surely would be consistent with this placement. Clearly they are an offshoot of the Carnivora and arguments can be provided to use either placement, a topic that is detailed sufficiently in Nowak (1999). For simplicity reasons, they are here still separated and believed to comprise 34 distinct species. This chapter groups several pinnipeds together, those of which placentas or information on placentas was available. It does not follow any specific order but the details are designated as best as they are available to me. The reader may also wish to read the chapter on walrus of this web site as there are overlaps.
Harbor seals are distributed essentially all over the world and travel long distances. It is apparent, however, that they return for breeding to the same region (“philopatry”). This feature led Stanley et al. (1996) to examine their possible mtDNA dissimilarities. It was found that, in general, significant differences do exist in various populations, with evidence of philopatry but also that the two populations of Atlantic and Pacific oceans have an ancient origin by splitting early; the cause of their divergence is discussed. In a recent FISH study of harbor seals, employing a human chromosome-specific library, Fronicke et al. (1997) found great conservation of pinniped, human and cat syntenic chromosomal regions. Wynen et al. (2001) deduced from a study of the cytochrome b gene that the separation of Otariinae from Arctocephalinae is not warranted and provided estimates for the times of their separation. Perry et al. (1995) have studied the possible relationships of seals in the Atlantic by mtDNA cytochrome b gene sequences and provided putative trees.
Obviously, the weights and sizes of these varied species are quite different one from another; they can easily be found in numerous reference, however (see Hayssen et al., 1993; Nowak, 1999). Male pinnipeds are generally larger than females. Neonatal weights also differ appreciably and males are then already larger and, in gray seals at least, the placenta of males is also larger (Boyd, 1990). All Pinnipedia have competent neonates with eyes wide open at birth. Some animals require access to rookeries on land or on ice. Longevity varies and it, as well as numerous other biologic and structural features has been detailed by Nishiwaki (1972) and Nowak (1999); in a California sea lion life extended to 30 years. Rates of maturation are dependent on many external factors such as number of males, total population, food resources, etc. and this aspect is discussed by Sergeant (1973).
There is an enormous literature on many common species of seals and sea lions that can be accessed readily by Google and through PubMed. It is too massive for inclusion here and only the main findings are referenced here.
 |
California sea lion at San Diego Zoo. |
 |
Sea lion rookery. |
 |
Swimming young California sea lion. |
2) General Gestational Data
The general aspects of reproduction in Pinnipedia have been reviewed by Costa & Crocker (1998). That chapter discusses the land-breeding and ice-breeding (pagophilic) species, their mating strategies, the maternal investment, breeding cycles, and other related topics. All species are believed to have an “embryonic diapause” (before implantation) of unknown length, and gestational length varies mostly from 8-9 months. Singleton births are the rule; twins are exceptional.
3) Implantation
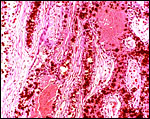
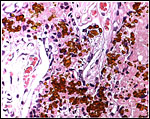
Virtually nothing is known of the implantation stages of the pinniped placenta, nor do we have precise knowledge of the “diapause” stage in Pinnipedia, a fact that Mossman (1987) much regretted. This is the case despite the frequency of captive sea lions and seals in aquaria and zoos. Mossman (1987) stated that some species of pinnipeds have a mesometrial, others an antimesometrial yolk sac location and that, in contrast to fissipedia, the saccular hematoma is less prominent. But this, too, varies greatly. (It is especially prominent in sea otters; see that chapter). As Harrison & Young (1966) have stated, “the large sausage-shaped chorionic sac completely fills the pregnant horn but does not enter the smaller sterile horn”. Also, “the labyrinth of the placental band is composed of numerous poorly marked lobules consisting of irregular and convoluted lamellae, enclosing thin-walled maternal vessels which are larger than in most carnivores.”
Small “vesicles” protrude on the fetal surface and are lined by cytotrophoblast; they may be white or contain detritus and blood. The marginal hematomas contain numerous yellow crystals, are Perl-stain negative by all investigators' studies, and show breakdown products of red blood cells electronmicroscopically in the cytotrophoblast. The latter makes up the cellular portions of this brown-red tissue. Harrison & Young (1966) went so far as to extract this tissue, searching for Fe ++ or Fe +++ , but finding none.
Remarkably, abdominal pregnancy may occur in pinnipeds with implantation on the peritoneum (Talent & Talent, 1975). While also rare, it does occur in the perhaps more invasive human placenta as well.
4) General Characterization of the Placenta
The placentas of male gray seals ( Halichoerus grypus ) were found to be larger than those associated with female fetuses (Boyd, 1990).
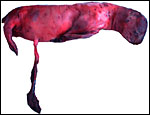
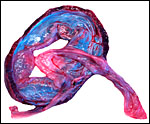
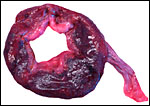
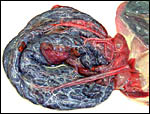
Northern Fur Seal ( Callorhinus ursinus ): This placenta was donated by Dr. T. Spraker and comes from a birth at St. Paul Island ( Alaska ). The circular, zonary placenta measured 40x15x1 cm and had an extensive hemophagous organ at the edges.
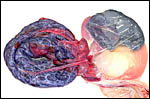
Harbor Seal ( Phoca vitulina ) : A single specimen was available from a stillborn at San Diego Zoo that was still contained within its membranes. It had a zonary placenta of 29x17 cm, and 0.5 cm thickness. A thin white film covered the maternal surface and at the borders brown hemophagous tissue was present. This was also found more focally beneath the chorion. The cord inserted at the center and was 10 cm long. The allantoic sac surrounded the avascular amnionic sac. Two 3 cm subchorionic cysts were present.
A second specimen was kindly made available by Dr. Judy St. Ledger. It came from a term pregnancy of a surviving pup, weighed 1,340 g, had the usual zonary shape which measured 50 cm in length and 14-24 cm in width, and which was 0.5-1 cm in thickness. The unusual feature of this placenta is that the amnionic cavity contained the already-shed lanugo hair (270g). These features are shown further below.
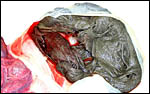
California Sea Lion ( Zalophus californianus ): A single placenta was available to me from an aborted female fetus that weighed 1,400 g and had a 32 cm CR length. The placenta weighed 80 g, was circular with a 4 cm breadth of the ring that had an overall measurement of 13 cm. The straight umbilical cord measured 17 cm and had four blood vessels. Additional slides of a term sea lion placenta are also displayed. A second specimen weighed 600 g, was 42 cm in circumferential size and 14-17 cm wide. It was 0.5 cm in thickness, dark red and had light red-yellow borders.
5) Details of fetal/maternal barrier
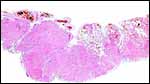
The fine structure of the implanted young seal placentas ( Lobodon carcinophagus and Hydrurga leptonyx ) was studied by Sinha & Erickson (1974). The electronmicroscopic appearance of the two species' placentas was similar and it can be assumed that this is true for most pinnipeds. They defined the placenta as being of a “lobulated, zonary, labyrinthine and endotheliochorial” structure. One difference among species may be the extent of the hemophagous zone, especially the presence or absence of the large projecting hematomas on the fetal surface. These authors showed the degenerating red blood cells but they did not find iron-staining in the hematomas, similar to our observations. Finely granular iron-positive deposits were seen at the trophoblastic basement membrane regions of the crabeater seal, but not in the leopard seal placentas of their study, much as is often but not always observed in immature human placentas. Harrison & Young (1966) did EM on gray seal and harbor seal placentas.
6) Umbilical cord
Harbor seal: The cord inserted in the center, was 10 cm long, non-spiraled and contained three large and numerous fine blood vessels and a widely patent allantoic duct. Starck (1957) stated that the cord of harbor seals measures 7.8 and 12 cm.
Grey seal: The umbilical cord was only 0.5 cm long but probably it was not all present in the specimen received.
California sea lion: The cord was 17 cm long in the prematurely aborted specimen shown above. It contained four large and numerous small vessels as well as the allantoic duct. There were no spirals.
7) Uteroplacental circulation
I have been unable to find any published reports.
8) Extraplacental membranes
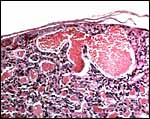
Yolk sacs are confined to the cord region and they appear to be transitory; they are only poorly identifiable in term placentas. The trophoblast of the chorionic membrane apposes the single layered epithelium of the uterine endometrium. The large allantois surrounds the amnionic sac completely and carries small blood vessels. Its connective tissue lies against the chorionic membrane and, on the central aspect, against the avascular amnion. The latter has a single layer of flat squamous epithelium without excrescences.
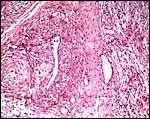
Harbor seal : There was marked chorionitis in this specimen but its cause was not ascertained.
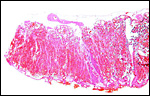
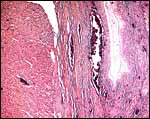
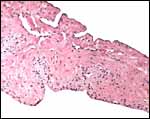 |
Harbor seal allantoamnion – amnion above. |
9) Trophoblast external to barrier
Invasion of trophoblast into the uterus has not been described in Pinnipedia and while syncytium is found, giant cells are absent. It should be noted that one abdominal pregnancy has been described, v.i. (Talent & Talent, 1975).
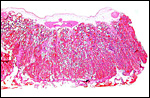
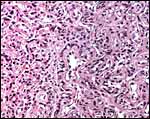
10) Endometrium
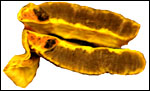
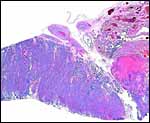
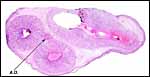
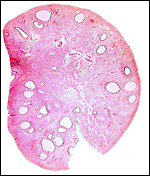
The endometrium of the unused or nonpregnant uterine horn shown in the next picture is characteristic of many seals in having “5-6 folds” (Sinha & Erickson, 1974). There is no decidualization.
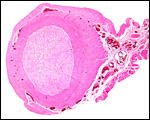 |
Cross section of gray seal uterus. |
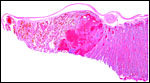
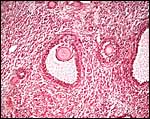
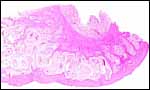 |
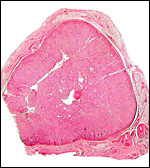
Uterus and endometrium of California sea lion ; note huge myometrial vasculature. This post partum animal died from generalized infection. |
11) Various features
Neither subplacenta nor metrial glands exist.
12) Endocrinology
Experimental exposure to increasing amounts of PCBs in harbor seals reduced the progesterone metabolism but increased testosterone metabolism (Troisi & Mason, 2000), suggesting to the authors a change in liver steroid biotransformation. The corpus luteum is exceptionally large, as has already been reported by others. It has a uniform cell population; its center is fibrotic in near term ovaries. All Pinnipedia that I have examined had a diffusely distributed “fetal interstitial gland” as was also described by Mossman & Duke (1973). The Graafian follicles near the corpus luteum of pregnancy in a northern fur seal had stimulated bands of theca interna; this region was thin in other developing follicles during nonpregnant phases of development. Adrenal glands are large and have an apparently uniform cellular makeup without much zonation evident, and certainly without pigmentation of the reticularis.
Hobson & Wide (1986) extracted the placentas of dolphin, sea lion, and seal and compared gonadotropin assays with extracts of human placentas. Mouse uterine weight changes, as well as immunoreactivity were studied. Apparently, sea lion and seal have a similar type of chorionic gonadotropin, albeit in a somewhat smaller amount for the seal. This approach was followed by Boyd (1990) who found the placentas of male gray seals to be heavier; the males also had earlier births but lower placental progesterone contents, while the gonadotropins were similar.
13) Genetics
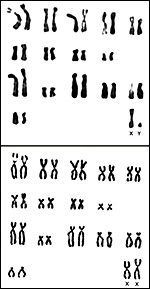
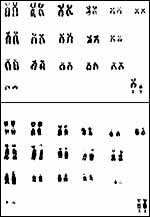
Fay et al. (1967), and especially Arnason (1972, 1974, 1977, 1981), have extensively compared various pinniped karyotypes. They essentially found only specimens with 32, 34 or 36 chromosomes. The conservation of a satellited marker chromosome of all carnivoran and pinnepedian species dates back to the presumed split late in Eocene (see Wurster & Benirschke, 1968). Many authors have suggested that evolution occurred mainly by Robertsonian fusion of acrocentrics, but additional fissioning was suggested by Perry et al. (1995). Several pinniped hybrids have been summarized by Gray (1972); a detailed study of an intergeneric hybrid [Harp Seal ( Phoca groenlandica ) x Hooded Seal ( Cystophora cristata )] comes from Kovacs et al. (1997). Kirchshofer (1968) described two (and depicted one) hybrids between Otaria byronia x Zalophus californianus . The delivery of the placenta was delayed and the 20 cm long cord was unusually thick. Schliemann (1968) reported on the apparently commoner hybridization between Arctocephalus pusillus x Zalophus californianus and referred to a few other repots of hybrids. Unfortunately, virtually nothing is known of their possible fertility.
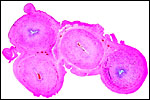
Harbor Seal: Phoca vitulina has 32 chromosomes; they are shown below (Hsu & Benirschke, 1973). The California sea lion has 36 chromosomes (Hsu & Benirschke, 1975), and the northern fur seal has 36 chromosomes as well (Hsu & Benirschke, 1969).
Acevedo-Whitehouse et al. (2003a) showed that inbreeding in harbor seals increases the susceptibility to infectious and parasitic diseases, as well as to neoplasia.
14) Immunology
I am not aware of any significant immunological studies.
15) Pathological features
There are numerous studies on various Pinnipedia regarding worm and virus infections, and especially, on the accumulation of toxic substances, organic and metallic. Additional information may be found in the chapter on walrus.
Griner (1983) summarized his large experience of necropsies done on beached animals (largely harbor seal and sea lion) in which he reviewed many parasitic infections, pox, bacterial infections and a variety of neoplasms (lymphosarcoma, squamous cell carcinoma of pharynx, and others). From the animals that he had obtained at the San Diego Zoo, he illustrated and identified many cases of foreign body accumulation in stomach and intestines. He also had several cases of renal lithiasis. Lyons et al. (2003) identified hookworms ( Uncinaria spp.) in sea lions as young as 2 days old, indicating a transmammary transmission. Infection rate was low in northern fur seals. Toxoplasma gondii & Neospora antibodies were found in a number of species by Dubey et al. (2003). Spraker et al. (2003) found ascarid gastric lesions in a large number of northern fur seals and other nematodes in lower frequency. Barlough et al. (1998) identified a reptilian calicivirus (Crotalus type 1) in several pinniped species off the coast of Oregon . Muller et al. (2003) described parapoxvirus infection in a harbor seal from the North Sea . Leptospira interrogans infection was diagnosed by Acevedo-Whitehouse et al. (2003b) in sea lion pups. Approximately one-half of blood samples of fur seals grew a variety of bacterial organisms (Vedros et al., 1982). Because of the frequency of recovery of heavy metals and organochlorides in pinnipeds, these animals are now considered to be good sentinel species. Beckmen et al. (2003) described that the content of organic chlorides and polychlorinated biphenyls perhaps related to poor immune responses. Lapointe et al. (1999) diagnosed acute placentitis in a harbor seal; it was the result of infection with Coxiella burnetii .
Gastric dilatation and volvulus was the cause of death in a fur seal kept in an aquarium (Frasca et al., 1996). A hepatic carcinoma with splenic metastases was seen in a stranded California sea lion (Acevedo-Whitehouse et al., 1999). Various stranded marine mammals were diagnosed as having renal disease, verminous pneumonia and dermatologic conditions (Gerber et al., 1993). Geraci (1974) identified thiamine deficiency in sea lions and gray seals; he proposed feeding supplements for the prevention. Premature birth associated with infection by the San Miguel Sea Lion Virus (like that of vesicular exanthema virus of swine) and Leptospira pomona isolated from placenta and pup was also related to higher levels of PCB and metals in California sea lions (Gilmartin et al., 1976). Northern fur seals suffered skull fractures and various neurologic hemorrhages from the method of clubbing during “harvest” (Jortner, 1974).
A most remarkable record of an abdominal pregnancy in a Steller sea lion comes from Talent & Talent (1975). The dead animal was beached and had a term male intrauterine fetus (12.5 kg) and, additionally, a fully-developed 16 kg fetus in the abdominal cavity. Its placenta had detached but from the facts that the uterus was intact and that there was marked hypervascularity near the lesser curvature of the stomach, implantation is likely to have been at that site. To my knowledge this is the only extrauterine pregnancy reported and it suggests that a specific interaction between trophoblast and endometrium is unnecessary for implantation.
16) Physiologic data
There is a very extensive consideration of all sorts of biological/physiological and pathological information found in Ridgway (1972) and in the other contributions found in this book. Hematology and chemistry reference values for harbor seals are also available from Morgan et al. (1998), and Lander et al. (2003). Biochemical data obtained from Australian sea lions were compared with those studies on the Californian species by Cargill et al. (1979).
The adaptation to diving and swimming was studied by electronmicroscopy of mitochondria and enzyme activity by Fuson et al. (2003). Jahan et al. (1991) determined the hemoglobin structure of the fur seal Arctocephalus galapagoensis .
17) Other resources
Cell lines of sea lion and harbor seal are available by contacting Dr. Oliver Ryder (oryder@uucsd.edu) at the San Diego Zoo, CRES division. Becker et al. (1990) described the tissue bank that stores tissue of marine mammals for future studies of trace elements and other toxic materials.
18) Other remarks – What additional Information is needed?
We have no information on preimplantation and early implantation stages, nor on the length of the diapause; thus, the true gestational length is unknown for all species. Endocrine data during gestation are only minimally available.
Acknowledgement
The specimen of Northern Fur seal was made available to me by Dr. Terry Spraker and the abortion specimen of the California sea lion was donated by Dr. Judy StLedger of Sea World, San Diego . She also kidnly provided the second sea lion and harbor seal placentas, as well as the photographs of the placenta with mass of shed hair. The first photograph of the California sea lion is from the San Diego Zoo collection, the two other photographs come though the courtesy of Dr. S. Ridgway.
References
Acevedo-Whitehouse, K., Constantino-Casas, F., Aurioles-Bambosa, D., Rodriguez-Martinez, H.A. and Godinez-Reyes, C.R.: Hepatic carcinoma with spleen metastasis in a California sea lion from the Gulf of California . J. Wildl. Dis. 35:565-568, 19999.
Acevedo-Whitehouse, K., Gulland, F., Greig, D. and Amos, W.: Inbreeding: Disease susceptibility in California sea lions. Nature 422:35, 2003a.
Acevedo-Whitehouse, K., de la Cueva, H., Gulland, F.M., Aurioles-Gamboa, D., Arellano-Carbajal, F. and Suarez-Guemes, F.: Evidence of Leptospira interrogans infection in California sea lion pups from the Gulf of California . J. Wildl. Dis. 39:145-151, 2003b.
Amoroso, E.C. and Matthews, L. H.: The foetal placenta and membranes of Halichoerus grypus . J. Anat. 86:487-488, 1952.
Arnason, U.: The role of chromosomal rearrangement in mammalian speciation with special reference to Cetacea and Pinnipedia. Hereditas 70:113-, 1972.
Arnason, U.: Comparative chromosome studies in Pinnipedia. Hereditas 76:179-225, 1974a.
Arnason, U.: Phylogeny and speciation in Pinnipedia and Cetacea – a cytogenetic study. Thesis, Lund, 1974b.
Arnason, U.: The relationship between the four principal pinniped karyotypes. Hereditas 87:227-242, 1977.
Arnason, U.: Localization of nucleolar organizing regions in pinniped karyotypes. Hereditas 94:29-34, 1981.
Barlough, J.E., Matson, D.O., Skilling, D.E., Berke, T., Berry , E.S., Brown, R.F. and Smith, A.W.: Isolation of reptilian calicivirus Crotalus type 1 from feral pinnipeds. J. Wildl. Dis. 34:451-456, 1998.
Becker, P.R., Koster, B.J., Wise , S.A. and Zeisler, R.: Alaskan marine mammal tissue archival project. Biol. Trace Elem. Res. 26-27:329-334, 1990.
Beckmen, K.B., Blake, J.E., Ylitalo, G.M., Stott, J.L. and O'Hara, T.M.: Organochlorine contaminant exposure and associations with hematological and humoral immune functional assays with dam age as a factor in free-ranging northern fur seal pups (Callorhinus ursinus). Mar. Pollut. Bull. 46:594-606, 2003.
Boyd, I.L.: Mass and hormone content of gray seal placentae related to fetal sex. J. Mammal. 71:101-103, 1990.
Cargill, C.F., Needham , D.J. and Judson, G.J.: Plasma biochemical values of clinically-normal Australian sea lions (Neophoca cinerea). J. Wildl. Dis. 15:105-110, 1979.
Costa, D.P. and Crocker, D.E.: Seals. In, Encyclopedia of Reproduction. Vol. 4, pp. 313-321. E. Knobil and J.D. Neill, eds. Academic Press, San Diego , 1998.
Dubey, J.P., Zarnke, R., Thomas, N.J., Wong, S.K., van Bonn, W., Briggs, M., Davis, J.W., Ewing, R., Mense, M., Kwok, O.C., Romad, S. and Thulliez, P.: Toxoplasma gondii, Neospora canium, Sarcocystis neurona and Sarcocystis canis -like infections in marine mammals. Vet. Parasitol. 116:275-296, 2003.
Fay, F.H., Rausch, V.R. and Feltz, E.T.: Cytogenetical comparison of some pinnipeds (Mammalia: Eutheria). Canad. J. Zool. 45:773-778, 1967.
Frasca, S. Jr., Dunn, J.L. and van Kruinigen, H.J.: Acute gastric dilatation with volvulus in a northern fur seal (Callorhinus ursinus). J. Wildl. Dis. 32:548-551, 1996.
Fronicke, L., Muller-Navia, J., Romanakis, K. and Scherthan, H.: Chromosomal homeologies between human, harbor seal (Phoca vitulina) and the putative ancestral carnivore karyotype revealed by Zoo-FISH. Chromosoma 106:108-113, 1997.
Fuson, A.L., Cowan, D.F., Kanatous, S.B., Polasek, L.K. and Davis , R.W.: Adaptations to diving hypoxia in the heart, kidneys and splanchnic organs of harbor seals (Phoca vitulina). J. Exp. Biol. 206:4139-4154, 2003.
Geraci, J.R.: Thiamine deficiency in seals and recommendations for its prevention. JAVMA 165:801-803, 1974.
Gerber, J.A., Roletto, J., Morgan, L.E., Smith, D.M. and Gage, L.J.: Findings in pinnipeds stranded along the central and northern California coast, 1984-1990. J. Wildl. Dis. 29:423-433, 1993.
Gilmartin, W.G., Delong, R.L., Smith, A.W., Sweeney, J.C., de Lappe, B.W., Risebrough, R.W., Griner, L.A., Dailey, M.D. and Peakall, D.B.: Premature parturition in the California sea lion. J. Wildl. Dis. 12:104-115, 1976.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough , England , 1972.
Griner, L.A. : Pathology of Zoo Animals. Zoological Society of San Diego , San Diego , California , 1983.
Harrison , R.J. and Young, B.A.: Functional characteristics of the pinniped placenta. In, Comparative Biology of Reproduction in mammals. Symposia London Zool. Society # 15, pp. 47-66. Academic Press, N.Y., 1966.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca , 1993.
Hernandez, M., Robinson, I. , Aguilar, A., Gonzalez, L.M., Lopez-Jurado, L.F., Reyero, M.I., Cacho, E., Franco, J., Lopez-Rodas, V. and Costas, E.: Did algal toxins cause monk seal mortality? Nature 393 (6680):28-29, 1998.
Hobson, B.M. and Wide, L.: Gonadotrophin in the term placenta of the dolphin (Tursiops truncatus ), the Californian sea lion (Zalophus californianus), the grey seal (Halichoerus grypus) and man. J. Reprod. Fertil. 76:637-644, 1986.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 3, Folio 131; Vol. 9, Folio 433; Vol. 7, Folio 338;. Springer-Verlag , New York , 1969, 1973, 1975.
Jahan, M., Ahmed, A., Trillmich, F. and Braunitzer, G.: The complete primary structure of the marine Carnivora, galapagoes fur seal ( Arctocephalus galapagoensis, Otariidae) hemoglobins. J. Protein Chem. 10:257-263, 1991.
Jortner, B.S.: Neuropathologic observations of head trauma in the northern fur seal. J. Wildl. Dis. 10:121-129, 1974.
Kirchshofer, R.: Notizen uber zwei Bastarde zwischen Otaria byronia (de Blainville) und Zalophus californianus (Lesson). Z. Säugetierk. 33:45-49, 1968.
Kovacs, K.M., Lydersen, C., Hammill, M.O., White, B.N., Wilson , P.J. and Malik, S.: A harp seal x hooded seal hybrid. Marine Mamm. Sci. 13:460-468, 1997.
Lander, M.E., Harvey, J.T. and Gulland, F.M.: Hematology and serum chemistry comparisons between free-ranging and rehabilitated harbor seal (Phoca vitulina richardsi) pups. J. Wildl. Dis. 39:600-609, 2003.
Lapointe, J.M., Gulland, F.M., Haines, D.M., Barr, B.C. and Duignan, P.J.: Placentitis due to Coxiella burnetii in a Pacific harbor seal (Phoca vitulina richardsi). J. Vet. Diagn. Invest. 11:541-543, 1999.
Lyons , E.T., DeLong, R.L., Spraker, T.R., Melin, S.R. and Tolliver , S.C. : Observations in 2001 on hookworms (Uncinaria spp.) in otariid pinnipeds. Parasitol. Res. 89:503-505, 2003.
Morgan, L.,, Kumaresan, S., Thomas, C. and MacWilliams, P.: Hematology and chemistry reference values for free-ranging harbor seals (Phoca vitulina) and the effects of hemolysis on chemistry values of captive harbor seals. J. Zoo Wildl. Med. 29:394-400, 1998.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison , Wisconsin , 1973.
Muller, G., Gröters, S., Siebert, U., Rosenberger, T., Driver, J., König, M., Becher, P., Hetzel, U. and Baumgärtner, W.: Parapoxvirus infection in harbor seals (Phoca vitulina) from the German North Sea. Vet Pathol. 40:445-454, 2003.
Nishiwaki, M.: General Biology. Chapter 1, pp. 3-204, in Mammals of the Sea. Biology and Medicine. C.C. Thomas, Springfield , IL , 1972.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Perry, E.A., Carr, S.M., Bartlett , S.E. and Davidson, W.S.: A phylogenetic perspective on the evolution of reproductive behavior in pagophilic seals of the Northwest Atlantic as indicated by mitochondrial DNA sequences. J. Mammal. 76:22-31, 1995.
Ridgway, S.H.: Homeostasis in the aquatic environment. Chapter 10, pp. 590-747, in Mammals of the Sea. Biology and Medicine. C.C. Thomas, Springfield , IL , 1972.
Schliemann, H.: Notiz uber einen Bastard zwischen Arctocephalus pusillus (Schreber, 1776) und Zalophus californianus (Lesson, 1828). Z. Sägetierk. 33:42-45, 1968.
Sergeant, D.E.: Environment and reproduction in seals. J. Reprod. Fertil. 19:555-561, 1973.
Sinha, A.A. and Erickson, A.W.: Ultrastructure of the placenta of Antarctic seals during the first third of pregnancy. Amer. J. Anat. 141:263-280, 1974.
Spraker, T.R., Lyons , E.T., Tolliver , S.C. and Bair, H.D.: Ascaridoid nematodes and associated lesions in stomachs of subadult male northern fur seals (Callorhinus ursinus ) on St. Paul Island , Alaska : (1987-1999). J. Vet. Diagn. Invest. 15:432-437, 2003.
Stanley , H.F., Casey, S., Carnahan, J.M., Goodman, S., Harwood, J. and Wayne , R.K.: Worldwide patterns of mitochondrial DNA differentiation in the harbor seal (Phoca vitulina). Mol. Biol. Evol. 13:368-382, 1996.
Starck, D.: Ueber die Länge der Nabelschnur bei Säugetieren. Z. Säugetierk. 22:77-86, 1957.
Talent, L.G. and Talent, C.L.: An extrauterine fetus in a Steller sea lion, Eumetopias jubata. Californ. Fish and Game 61:233-234, 1975.
Troisi, G.M. and Mason, C.F.: PCB-associated alteration of hepatic steroid metabolism in harbor seals (Phoca vitulina). J. Toxicol. Environm. Health A 61:649-655, 2000.
Vedros, N.A., Quinlivan, J. and Cranford , R.: Bacterial and fungal flora of wild northern fur seals (Callorhinus ursinus). J. Wildl. Dis. 18:447-456, 1982.
Wozencraft, W.C.: Order Carnivora. Pp. 279-348, in: Mammal Species of the World. A Taxonomic and Geographic Reference. D.E. Wilson and D.M Reeder, eds. 2 nd Edition. Smithsonian Institution Press, Washington, 1992.
Wurster, D.H. and Benirschke, K.: Comparative cytogenetic studies in the Order Carnivora. Chromosoma 24:336-382, 1968.
Wynen, L.P., Goldsworthy , S.D. , Insley, S.J., Adams , M., Bickham, J.W., Francis, J., Gallo, J.P., Hoelzel, A.R., Majluf, P., White, R.W. and Slade, R.: Phylogenetic relationships within the eared seals (Otariidae:Carnivora): implications for the historical biogeography of the family. Mol. Phylogenet. Evol. 21:270-284, 2001.