|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
Cervus unicolor niger
Order: Artiodactyla
Family: Cervidae
1) General Zoological Data
In most classifications, Sambar deer belong to the subgenus Rusa. Both are Hindi names for deer. Sambars are widely distributed through India, South-East Asia and many Pacific Islands and have been introduced to Australia and New Zealand (Wilson & Reeder, 1992). Some of the islands may also have sambars that were recently introduced. The subgenus (Rusa) has four species (C. alfredi, C. marianus, C. unicolor, C. timorensis). The German designation often used is "Aristotlehirsch", since the animal was already well-known to Aristotle from West India. It was then also designated as being characteristically a six-pointer. In German, these animals are also referred to as "Pferdehirsch" because of their long legs. A recent study of mtDNA by Randi et al. (2001) suggests that this clade separated from other cervidae around 5 MYA. Nowak (1999) referred to recent electrophoretic findings that have suggested a close relationship to Axis and Dama deer. Haltenorth (1968) differentiated three species, with 18 subspecies. Adult males are larger than females and have characteristically 6 antler points and long legs. Their weights vary from 100-315 kg. Sambars are reported to be more of a browsing than grazing animal that prefers to live in wooded regions. Newborns weigh around 10 kg. The longevity in captivity is at least 24 years and 5 months (Jones, 1993).
Because of the availability of this specimen and the large size of its placental cotyledons, this deer species is treated here separately; other cervid species are detailed in a separate chapter, on "Deer species" (see there for more discussion).
 |
Indian sambar deer group at San Diego Wild Animal Park. |
 |
Malayan sambar deer stag at San Diego Zoo. |
 |
Malayan sambar hind with fawn at San Diego Wild Animal Park. |
The length of gestation is 8 months or 240 days (Nowak, 1999; Hayssen al., 1993) with usually single offspring. Records of twins exist, but they occur uncommonly (Hayssen et al., 1993). Plotka (1999) referred to studies that indicate that the length of gestation is 240-270 days.
3)
Implantation
I have had the opportunity of studying one pregnant uterus near the end
of gestation. This was from a hind that died from intraspecific trauma.
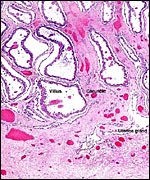
The uterus measured 130 cm in length; the female fetus weighed 8.3 kg
and had a crown-to-rump length of 66 cm. There were six large cotyledons,
three in each uterine horn. The cotyledons weighed from 300 to 320 g (with
their caruncle attached) and measured 5 x 9 cm. The uterus is bicornuate
and the placenta implants on the three mesometrial caruncles of both horns.
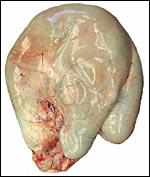
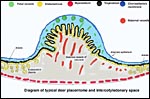 |
Diagram of typical deer placentome and intercotyledonary space. |
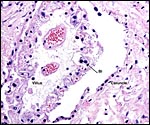
As other deer species, sambars have only a small number of caruncles and, therefore, cotyledons. This specimen had six cotyledons that were of rather large size. This is thus an "oligo-cotyledonary", epithelio-chorial placenta without invasion into the maternal tissue, much like that of most other cervidae studied (see Hamilton et al., 1960). The cotyledons have a convex shape.
The microscopic details of the cotyledonary and arcade structures of the sambar deer placenta are most similar to the other deer species studied. They have been summarized in detail in publications by Sinha et al. (1969) and by Hamilton et al. (1960). Most of this is reviewed in some detail in the other general chapter on "Deer Species" to which the reader is referred. This placenta is mostly remarkable because of the large size of the cotyledons. They are so large that a complete cross section cannot be fitted onto one slide. Binucleate trophoblast are present as in other ruminants; they PAS-positive and one has to assume that they also produce relaxin or lactogen as other ungulates, although that has not been shown for this species. The fetal capillaries course within the trophoblastic cover of the villi.
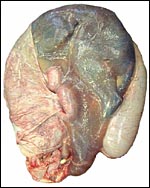
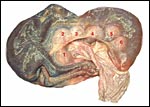
The umbilical cord of this pregnancy measured 7 cm in length, had four blood vessels and a large, patent allantoic duct. The cord was not spiraled. The surface of the umbilical cord had large stretches of massive squamous metaplasia, as shown below. The amnionic epithelium had fine granules on its surface, also consisting of squamous metaplasia. The allantoic duct shown below was lined by urothelium and was empty. It was accompanied by a large number of small blood vessels.
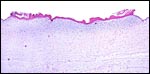 |
The surface of the umbilical cord has long stretches of keratinized epithelium. |
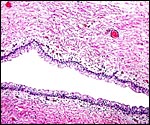 |
Allantoic duct in the center of umbilical cord with small vessels. |
I know of no studies in this deer species.
8)
Extraplacental membranes
The allantoic sac was filled with milky, yellow fluid and had a very thin
epithelium. There were no hippomanes. The amnion had many tiny foci of
squamous metaplasia. They are composed of cells that were described in
detail for the white-tailed deer by Sinha et al. (1970).
9) Trophoblast external to barrier
There is no trophoblastic infiltration of uterus or caruncle.
10) Endometrium
The bicornuate uterine horn has three mesometrially located caruncles
in each horn, most similar to that described in detail by Sinha et al.
(1969) for white-tailed deer.
11) Various features
No special features were identified.
12)
Endocrinology
I have had the opportunity of examining also the fetal testes of sambar
deer. Their interstitial cell compartment appears to be completely unstimulated,
from which I infer that no fetal gonadotropins are active. The presence
of binucleate trophoblastic cells on the villi suggests relaxin or lactogen
production during pregnancy (Wooding et al., 1997). Although no specific
studies on Rusa are included, Plotka (1999) summarized most of
what is known of the endocrine function in cervidae.
13)
Genetics
Cooper & Herbert (2001) examined the survival of animals derived from
the small introduced population of sambar that now inhabit Australia and
New Zealand. Remarkably, a thriving colony of sambar deer was founded
by only two animals.
Gray (1972) does not list any hybridization with other species. Putative
hybrids with Cervus timorensis (a closely related species) have
not been definitely characterized (Dratch & Pemberton, 1991). These
authors studied electrophoretic characters of deer species but stated
that no Sambar deer had been studied by then.
Their chromosome picture is confusing. Chandra et al. (1967) found an
Indian sambar deer from Mysore, India, to have 58 chromosomes. Animals
from the Philippines karyotyped by us had 64 or 65 chromosomes. The NF,
however, has always been 70, as is the case in most deer species (Hsu
& Benirschke, 1973). There is much unresolved confusion of Cervus
unicolor and Rusa species that is also evident from the review
by Groves and Grubb (1987). Among other remarks, they cited Wang &
Du (1982) as having identified the Chinese Cervus (Rusa) unicolor dejeani
(cambojensis) as having 2n=62 chromosomes, with NF 70. Halnan (1989)
listed the following for Cervus unicolor: 2n= 58, 64/65, 62. Our
experience with Cervus unicolor malaccensis is 2n=56; for C.
unicolor niger it was 2n=58. A male "Sambar deer" described
by Neitzel (1979) from the Berlin Zoo had 2n=60. It is thus apparent that
extensive Robertsonian rearrangements have occurred in this subgenus that
needs more extensive study of animals with known, precise origin of location.
The NF (nombre fondamental - chromosome arms), however, is always 70.
Miyamoto et al. (1990) studied some mtDNA sequences of cervinae and found
close relation of muntjacs and sambars.
14)
Immunology
I know of no studies in this deer species.
15)
Pathological features
With advanced age, sambar deer have developed arthritis that necessitated
euthanasia (Griner, 1983). According to the review by Heuschele et al.
(1984), Rusa (Cervus timorensis) have been reported to suffer
from malignant catarrhal fever.
16)
Physiologic data
I know of no studies in this deer species.
17)
Other resources
Numerous cell strains of various sambar deer are available from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other remarks - What additional Information is needed?
More endocrine and chromosomal data are needed, especially of what are
now considered to be "subspecies". It appears from the various
chromosomal studies that some of the "subspecies" have widely
divergent chromosome numbers and thus may deserve species ranking. In
addition, early gestational stages and descriptions of term delivered
placentas would be of interest.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Chandra, H.S., Hungerford, D.A. and Wagner, J.: Chromosomes of five artiodactyl
mammals. Chromosoma 21:211-220, 1967.
Cooper, D.W. and Herbert, C.A.: Genetics, biotechnology and population management of over-abundant mammalian wildlife in Australasia. Reprod. Fertil. Dev. 13:451-458, 2001.
Dratch, P.A. and Pemberton, J.M.: Application of biochemical genetics to deer management: What the gels tell. Chapter 87, pp.367-389, in: The Biology of Deer, R.D. Brown, ed. Springer-Verlag, N.Y., 1991.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Groves, C.P. and Grubb, P.: Relationships of living deer. Pp. 21-59, in Biology and Management of the Cervidae. C.M. Wemmer, ed. Smithsonian Institution Press, Washington, D.C. 1987.
Halnan, C.R.E., ed.: Cytogenetics of Animals. CAB International, Oxford, 1989.
Haltenorth, Th.: Hirsche. Chapter 8 (pp. 168-272) in, Grzimeks Tierleben, Volume 13. Kindler Verlag, Zürich, 1963.
Hamilton, W.J., Harrison, R.J. and Young, B.A.: Aspects of placentation in certain cervidae. J. Anat. 94:1-33, 1960.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Heuschele, W.P., Oosterhuis, J., Anderson, M.P., Swansen, M. and Fletcher, H.R.: Malignant catarrhal fever in wild ruminants. Chapter 25 (pp. 296-308) in, One Medicine, O.A. Ryder and M.L. Byrd, eds. Springer-Verlag N.Y., 1984.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Folio 344, Vol. 7 1973. Springer-Verlag, N.Y.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Miyamoto, M.M., Kraus, F. and Ryder, O.: Phylogeny and evolution of antlered deer determined from mitochondrial DNA sequences. Proc. Natl. Acad. Sci. 87:6127-613, 1990.
Neitzel, H.: Chromosomenevolution in der Familie der Hirsche (Cervidae). Bongo 3:27-38, 1979
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Plotka, E.D.: Deer. Pp 842-857, in, Encyclopedia of Reproduction. E. Knobil and J.D. Neill, eds. Vol. 1, Academic Press, San Diego, 1999.
Randi, E., Mucci, N., Claro-Hergueta, F., Bonnet, A. and Douzery, J.P.: A mitochondrial DNA control region phylogeny of the Cervinae: speciation in Cervus and implications for conservation. Anim. Conser. 4:1-11, 2001.
Sinha, A.A., Seal, U.S., Erickson, A.W. and Mossman, H..: Morphogenesis of the fetal membranes of the white-tailed deer. Amer. J. Anat. 126:201-241, 16.
Sinha, A.A., Seal, U.S. and Erickson, A.W.: Ultrastructure of the amnion and amniotic plaques of white-tailed deer. Amer. J. Anat. 127:369-396, 1970.
Wang, Z. and Du, R.: Evolution of karyotype of the genus Cervus. Acta Genet. Sinica 9:24-31, 1982 (in Chinese).
Wilson D.E. and Reeder, Eds.: Mammal Species of the World. Second Ed. Smithsonian Institution Press, Washington, DC, 1992.
Wooding, F.B., Morgan, G. and Adam, C.L.: Structure and function in the ruminant synepitheliochorial placenta: central role of the trophoblast binucleate cell in deer. Microsc. Res. Tech. 38:88-99, 1997.