|
(Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.)
|
| Last updated: July 10, 2004. |
Pithecia pithecia pithecia
Order: Primates
Family: Cebidae (Pitheciidae)
1) General Zoological Data
Of the five species of saki monkeys now recognized by Nowak (1999), this species has the most northern distribution. See Hershkovitz, (1979, 1987) for recent taxonomic considerations. Saki is a Tupi Indian designation for this monkey (Gotch, 1979). The saki is one species of the large infraorder of Platyrrhini that has been divided by various molecular techniques into three genera, the Pitheciidae, Atelidae and Cebidae (Harada et al., 1995; Schneider et al., 2001). Steiper & Ruvolo (2003) added to the analysis of DNA sequences of the X-linked G6PD in these species. Adults weigh 700-1,700 (?2,500)g and have a life expectancy of 35 years (Nowak, 1999). Single young are born after a gestation of 163-176 days (Eisenberg, 1989). Sakis are not endangered now and are only occasionally seen in zoos. They are well-known, however, for their leaping ability in the forests. There is a significant facial difference in the sexes.
Rosenberger (1992) suggested that sakis depend primarily on seeds for their protein needs, a fact supported by the experiments and descriptions of Brumloop et al. (1994). They are also known to consume small mammals (bats) and birds.
 |
Male saki monkey at San Diego Zoo. |
 |
Female saki monkey at San Diego Zoo. |
The length of gestation is described as being 163-176 days (Eisenberg, 1989) and produces a single quite mature young. Twins have not been described.
3)
Implantation
No early stages have been described of this species. Placentation occurs
anteriorly and posteriorly in the single uterus.
4)
General Characterization of the Placenta
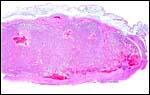
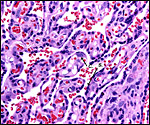
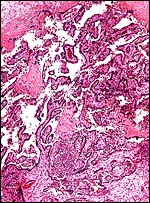
This placenta has not been described earlier. It is a bilobed, villous
to trabecular hemochorial organ with great similarity to the placenta
of spider monkeys. It comes from a term pregnancy at the San Diego Zoo
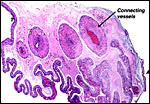
with a surviving infant. The placenta is bilobed with cord insertion on
a smaller lobe and connecting blood vessels between the two lobes. It
weighed 24.5 g and the lobes measured 4x4x0.5 cm and 4x4.5x0.5 cm. The
cord measured 14 cm in length and 0.3 cm in width. The fetal surface was
slightly greenish (?meconium), the maternal surface was smooth and had
fine dots of yellow color (dystrophic calcifications). Cross sections
showed a very dark, compact tissue without infarcts.
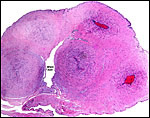
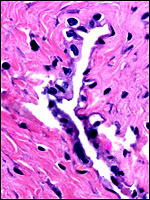
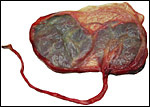 |
Term placenta of two lobes with small connecting blood vessels. |
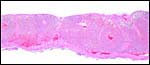
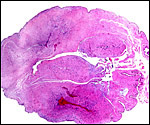
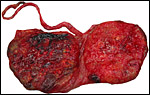 |
Maternal surface of the same placenta. |
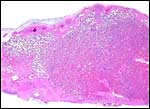
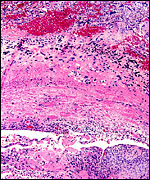
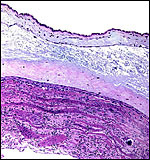
The villous tissue is quite uniform and different from that of Callithricidae. It is somewhat trabecular and mostly villous. Long connecting strands of connective tissue extend from the chorion to the floor of the placenta. Villi are typical of primates, with a diffuse syncytial cover, few syncytial giant cells in the intervillous space, prominent villous cytotrophoblast beneath the syncytium, and a few islands of extravillous trophoblast. The latter and some of the fibrinoid material have small foci of calcification, especially at the placental floor. In contrast to marmosets and tamarins, there is no extramedullary hematopoiesis in the villi. The villous connective tissue is very sparse and only very rare Hofbauer cells are present.
The placental floor has rather thick layers of fibrin and fibrinoid with mild infiltration of cytotrophoblast, but it does not extend deeply into the decidua basalis.
There is virtually no infiltration of trophoblast into the maternal vasculature.
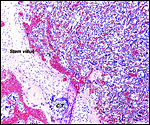
The umbilical cord was 14 cm long and 0.3 cm wide. It inserted near the margin and had no spirals. The umbilical cord possesses four large blood vessels, two arteries and two veins, but no small vessels. In addition, the cord has a large allantoic duct, similar to that of the spider monkey (Miller & Benirschke, 1985). There is no surface squamous metaplasia. In general, the findings are extremely similar to those of the spider monkey (see chapter on Ateles), except for the apparent lack of an allantoic sac.
No studies have been conducted but there is virtually no modification of the maternal spiral arterioles by invasive trophoblast.
8)
Extraplacental membranes
The amnion on the surface of the placenta was very delicate and it was
stained green. No meconium was found, however. Thus, the discoloration
may be the result of the edema present. There was some subamnionic edema,
perhaps secondary to the long post delivery interval. The chorion carried
the large interlobar blood vessels. The undersurface of the chorion is
invested by cytotrophoblast and it infiltrates slightly into the sparse
decidua capsularis. There are no atrophied villi within the free membranes.
The decidua capsularis showed mild degenerative changes. Despite the presence
of a large allantoic duct in the umbilical cord, I was unable to find
an allantoic sac.
There is superficial infiltration of the basal endometrium by extravillous trophoblast, but without giant cell formation. Very sparse vascular infiltration occurs, unlike that found in the spider monkey; there one finds much more trophoblastic infiltration (see chapter on Ateles).
10)
Endometrium
There is very, very sparse decidual transformation of the endometrium
and little infiltration by trophoblast.
11)
Various features
None.
12)
Endocrinology
I am not aware of any studies in this species.
13)
Genetics
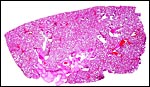
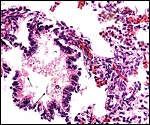
Sakis have 48 chromosomes, as shown in the next picture (Hsu & Benirschke,
1977). The karyotype has been well described by de Boer (1975) and this
karyotype comes from him. Two hybrids between different saki species are
listed by Gray (1972).
Porter et al. (1999) assessed globin gene sequences of Callicebus torquatus
and Pithecia irrorata. rDNA carrying chromosomes of numerous primate
species, including South American species and P. pithecia were
studied by Henderson et al. (1977). Alpha satellite DNA studies were conducted
by Alves et al. (1994). The separation of platyrrhines from catarrhines
was delineated by the study of gamma-globin genes of Chiu et al. (1996).
 |
Male chromosome sets of Saki monkeys. |
 |
Female chromosome sets of Saki monkeys. |
I am not aware of any studies.
15)
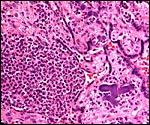
Pathological features
Scott (1992) depicts extramedullary hematopoiesis in the liver and aspirated
squames in the lung of stillborn sakis, both normal findings. Dirofilariasis
affected a saki at Dallas Zoo (Gamble et al., 1998). In Florida, a saki
developed abdominal lesions that were ultimately diagnosed as being due
to Mycobacterium avium lymphadenitis (Heard et al., 1997). Toxoplasmosis
was identified in tamarins and sakis by Dietz et al. (1997). Malaria parasites
(Plasmodium brasilianum) were found by Fandeur et al. (2000).
A fetal demise of a near-term Northern White-faced Saki fetus (117 g) was due to infection with Yersinia enterocolitica organisms that were also cultured from the fetus. What is remarkable in this case is the fact that villous abscesses were present, in addition to fetal septicemia with small abscesses in lung, liver, adrenal, kidney but that there was no evidence of chorioamnionitis, deciduitis or inflammation in the maternal floor of the placenta. In fact, the most severe inflammatory reaction occurred in the villi of the nearly mature placenta. The mother was asymptomatic and survived another four years during which she had one abortion. Eventually, euthanasia was performed because of terminal renal disease and infestation with Gongylonema parasites. It remains unknown how the organisms entered the fetus without known maternal illness or therapy.
16)
Physiologic data
Norconk et al. (2002) examined the transit time of ingesta (seeds etc.)
of sakis and the animal's adaptation to much longer transit times than
found in other cebid species. Boissinot et al. (1998) found the X-linked
color vision system of sakis to differ markedly from other Platyrrhini.
The patterns of retinal radiations of platyrrhine species was examined
by Kaas et al. (1978). Scent marking was studied by Brumloop et al. (1994).
The cellular vitamin D receptors were examined in considerable detail
by Gacad & Adams (1991) to explain the differences of vitamin D needs
of South American primates.
17)
Other resources
Cell strains of this species are available from CRES
by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
Early stages of placentation and endocrine studies are needed.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Alves, G., Seuanez, H.N. Fanning, T.: Alpha satellite DNA in neotropical
primates (Platyrrhini). Chromosoma 103:262-267, 1994.
De Boer, L.E.M.: The somatic chromosome complement and the ideogram of Pithecia pithecia pithecia (Linnaeus, 1766). Folia primatol. 23:149-157, 1975.
Boissinot, S., Tan, Y., Shyue, S.K., Schneider, H., Sampaio, I., Neiswanger, K., Hewett-Emmett, D. and Li, W.H.: Origins and antiquity of X-linked triallelic color vision systems in New World monkeys. Proc. Natl. Acad. Sci. USA 95:13749-13754, 1998.
Brumloop, A., Homburg, I., Peetz, A. and Riehl, R.: Gular scent glands in adult female white-faced saki, Pithecia pithecia pithecia, and field observations on scent-marking behaviour. Folia Primatol. 63:212-215, 1994.
Chiu, C.H., Schneider, H., Schneider, M.P., Sampaio, I., Meireles, C., Slighton, J.L., Gumucio, D.L. and Goodman, M.: Reduction of two functional gamma-globin genes to one: an evolutionary trend in New World monkeys (infraorder Platyrrhini). Proc. Natl. Acad. Sci. USA 93:6510-6515, 1996.
Dietz, H.H., Henriksen, P., Bille-Hansen, V. and Henriksen, S.A.: Toxoplasmosis in a colony of New World monkeys. Vet. Parasitol. 68:299-304, 1997.
Eisenberg, J.F.: Mammals of the Neotropics: the Northern Neotropics. University of Chicago Press, 1989.
Fandeur, T., Volney, B., Peneau, C. and de Thoisy, B.: Monkeys of the rainforest in French Guiana are natural reservoirs for P. brasilianum/P. malariae malariae. Parasitology 120:11-21, 2000.
Gacad, M.A. and Adams, J.S.: Endogenous blockade of 1,25-dihydroxyvitamin D-receptor binding in New World primate cells. J. Clin. Inv. 87:996-1001, 1991.
Gamble, K.C., Fried, J.J. and Rubin, G.J.: Presumptive dirofilariasis in a pale-headed saki monkey (Pithecia pithecia). J. Zoo Wildl. Med. 29:50-54, 1998.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Harada, M.L., Schneider, H., Schneider, M.P., Sampaio, I., Czelusniak, J. and Goodman, M.: DNA evidence on the phylogenetic systematics of New World monkeys: support for sister-grouping of Cebus and Saimiri from two unlinked nuclear genes. Mol. Phylogenet. Evol. 4:331-349, 1995.
Heard, D.J., Ginn, P.E. and Neuwirth, L.: Mycobacterium avium-intracellulare infection in a white-faced saki (Pithecia pithecia). J. Zoo Wildl. Med. 28:185-188, 1997.
Henderson, A.S., Warburton, D., Megraw-Ripley, S. and Atwood, K.C.: The chromosomal location of rDNA in selected lower primates. Cytogenet. Cell Genet. 19:281-302, 1977.
Hershkovitz, P.: The species of sakis, genus Pithecia (Cebidae, Primates), with notes on sexual dichromatism. Folia Primatol. 31:1-22, 1979.
Hershkovitz, P.: The taxonomy of South American sakis, genus Pithecia (Cebidae, Platyrrhini): a preliminary report and critical review with the description of a new species and a new subspecies. Amer. J. Primatol. 12:387-468, 1987.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 10, Folio 515, 1977. Springer-Verlag, New York.
Kaas, J.H., Huerta, M.F., Weber, J.T. and Harting, J.K.: Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J. Comp. Neurol. 182:517-553, 1978.
Miller,
P.W. and Benirschke, K.: A large allantoic sac in the placenta of the
spider
monkey Ateles geoffroyi. Placenta 6:423?426, 1985.
Norconk, M.A., Oftedal, O.T., Power, M.L., Jakubasz, M. and Savage, A.: Amer. J. Primatol. 58:23-34, 2002.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Porter, C.A., Czelusniak, J., Schneider, H., Schneider, M.P., Sampaio, I. and Goodman, M.: Sequences from the 5'flanking region of the epsilon-globin gene support the relationship of Callicebus with the pitheciins. Amer. J. Primatol. 48:69-75, 1999.
Rosenberger, A.L.: Evolution of feeding niches in New World monkeys. Amer. J. Phys. Anthropol. 88:525-562, 1992.
Schneider, H., Cavanez, F.C., Sampaio, I., Moreira, M.A., Tagliaro, C.H. and Seuanez, H.N.: Can molecular data place each neotropical monkey in its own branch? Chromosoma 109:515-523, 2001.
Scott, G.B.D.: Comparative Primate Pathology. Oxford University Press, 1992.
Steiper, M.E. and Ruvolo, M.: New World monkey phylogeny based on X-linked G6PD DNA sequences. Mol. Phylogenet. Evol. 27:121-130, 2003.