| |
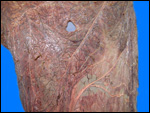
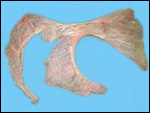
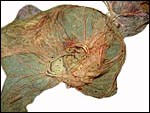
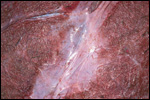
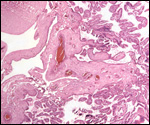
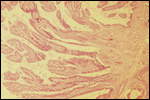
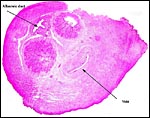
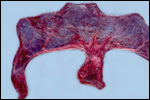
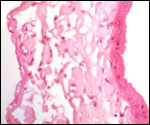
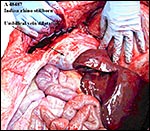
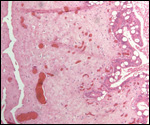
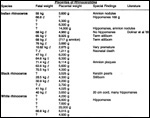
Since then we have seen two more placentas of Indian rhinoceroses. One weighed
7,000 g, had a healthy newborn, had hippomanes and measured 304 cm in greatest
length, 132 and 54 cm in largest and smallest diameters and had a 10 cm
cord attached. The other also was at term, had a healthy neonate and weighed
6,600 g. It measured 270 x 100 cm and had a 10 cm cord attached.
Combined
References
Cell strains of four species of rhinoceros are available from the “Frozen zoo” at the Zoological Society of San Diego: www.FrozenZoo@sandiegozoo.org
Amoroso,
E.C.: Placentation. In, Marshall's Physiology of Reproduction. A.S. Parkes,
ed., 3rd ed., Vol. II. London. Longmans, Green, 1952.
Ashley,
M.V., Melnick, D.J. and Western, D.: Conservation genetics of the black
rhinoceros (Diceros bicornis): I. Evidence from the mitochondrial
DNA of 3 populations. Conserv. Biol. 1:71-77, 1990.
Baumgartner,
K. and Schaftenaar,W.: Fecal progesterone, estrogen, and androgen metabolites
for nonivasive monitoring of reproductive function in the female Indian
rhinoceros, Rhinoceros unicornis. Gen. Compar. Endocrinol. 119:300-307,
2000.
Benirschke,
K. and Calle, P.P.: The placenta of the Beluga whale (Delphinapterus
leuca). Verh. Ber. Erkg. Zootiere 36:309-314, 1994.
Benirschke,
K. and Lowenstine, L.J.: The placenta of the rhinocerotidae. Verh. Ber.
Erkr. Zootiere (Dresden). 37:15-23, 1995.(Attached at end)
Bennett,
C. and Kleiman, D.G.: Black rhinoceros (Diceros bicornis) in U.S. Zoos:
II. Behavior, breeding success, and mortality in relation to housing facilities.
Zoo Biol. 18:35-52, 1999.
Carlstead,
K., Fraser, J., Bennett, C. and Kleiman, D.G.: Black rhinoceros (Diceros
bicornis) in U.S. Zoos: II. Behavior, breeding success, and mortality
in relation to housing facilities. Zoo Biol. 18:35-52, 1999.
Chapin,
H., Malecek, A.C., Miller, R.E., Bell, C.E., Gray, L.S. and Hunter, V.L.:
Acute intravascular hemolytic anemia in the black rhinoceros: Hematologic
and immunohematologic observations. Amer. J. Vet. Med. 47:1313-1320, 1986.
Dixon,
H.G. and Robertson, W.B.: The growth of the conceptus and its blood supply.
In, Foetus and Placenta. Klopper, A. and E. Diczfalusy, eds. Oxford and
Edinburgh: Blackwell, pp. 1-32, 1969.
Dolinar,
Z.J., Ludwig, K.S. und Müller, E.: Ein weiterer Beitrag zur Kenntnis
der Placenten der Ordnung Perissodactyla: Zwei Geburtsplacenten des Indischen
Panzernashorns. (Rhinoceros unicornis L.). Acta Anat. 61:331-354,
1965.
Galama, W.T., Graham, L.H. and Savage, A.: Comparison of fecal storage methods for steroid analysis in black rhinoceros (Diceros bicornis). Zoo Biol. 23:291-300, 2004.
George,
M. Jr., Chemnick, L.G., Cisova, D., Gabrisova, E., Stratil, A. and Ryder,
O.A.: Genetic differentiation of white rhinoceros subspecies: diagnostic
differences in mitochondrial DNA and serum proteins. In, Proc. Intern.
Conference on Rhinoceros Biology and Conservation, San Diego, CA 1991,
pp. 105-113.
Groves, C.P.: Ceratotherium simum. In, Mammalian Species. 8:1-6, 1972.
Amer. Soc. Mammalogy.
Groves, C.P.: Taxonomic notes on the white rhinoceros. Ceratotherium
simum (Burchell, 1817). Säugetierk. Mitteil. 23:200-212, 1975.
Groves, C.P.: Phylogeny of the living species of Rhinoceros. Z. zool.
System. Evol. 21:293-313, 1983.
Hansen,
K.M.: Q-bands of some chromosomes of white rhinoceros (Diceros simus).
Hereditas 82:205-208, 1976.
Harley,
E.H. and O'Ryan, C.: Molecular genetic studies of southern African rhinoceros.
In, Proc. Intern. Conference on Rhinoceros Biology and Conservation, San
Diego, CA 1991, pp. 101-104.
Heinichen,
I.G.: Karyological studies on southern African perissodactyla. Kodoe 13:51-108,
1970.
Houck,
M.L., Ryder, O.A., Váhala, J., Kock, R.A. and Oosterhuis, J.E.:
Diploid chromosome number and chromosomal variation in the white rhinoceros
(Ceratotherium simum). J. Hered. 85:30-34, 1994.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 7: Folio 340, 1973. Springer-Verlag, New York.
Hungerford,
D.A., Chandra, H.S. and Snyder, R.L.: Somatic chromosomes of a black rhinoceros
(Diceros bicornis Gray 1821). Amer. Naturalist 101:357-358, 1967.
Kloosterman,
G.J. and Huidekoper, B.L.: The significance of the placenta in obstetrical
mortality. A study of 2.000 births. Gynaecologia 138:529-550, 1954.
Lang,
E.M.: Geburt eines Panzernashorns, Rhinoceros unicornis, im Zoologischen
Garten Basel. Säugetierk. Mitt. 5:69-70, 1957.
Lang,
E.M.: Einige biologische Daten vom Panzernashorn (Rhinoceros unicornis).
Rev. Suisse Zool. Genève 74:603-607, 1967.
Laurie,
W.A., Lang, E.M. and Groves, C.P.: Rhinoceros unicornis. In, Mammalian
Species # 211, pp.1-6, 1983. Amer. Soc. Mammalogy.
Ludwig,
K.S.: Zur Kenntnis der Geburtsplacenten der Ordnung Perissodactyla. Acta
Anat. 49:154-167, 1962.
Ludwig, K.S. und Villiger, W.: Zur Ultrastruktur der Blattzottenepithelien
in der Placenta des Indischen Panzernashorns (Rhinoceros unicornis
L.). Acta Anat. 62:593-605, 1965.
Ludwig,
K.S. und Müller, E.: Zur Histochemie der Placenta des Panzernashorns
(Rhinoceros unicornis L.). Acta Anat. Suppl. 115:155-159, 1965.
Mereniender,
A.M., Woodruff, D.S., Ryder, O.A., Kock, R. and Váhala, J.: Allozyme
variation and differentiation in African and Indian rhinoceroses. J. Hered.
80:377-382, 1989.
Miller,
R.E.: Veterinary Bibliography for Rhinoceros. A.A. Balkema Publ., Amsterdam,
1983.
Miller,
R.E. and Boever, W.J.: Fatal hemolytic anemia in the black rhinoceros:
Case report and a survey. J.A.V.M.A. 181:1228-1231, 1982
Morales,J.C.,
Andau, P.M., Supriatna, J., Zainuddi, Z.Z. and Melnick, D.J.: Mitochondrial
DNA variability and conservation genetics of the Sumatran rhinoceros.
Conserv. Biol. 11:539-543, 1997.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan Company. Houndmills, 1987.
Naaktgeboren,
C. and Zwillenberg, H.H.L.: Untersuchungen über die Auswüchse
am Amnion und an der Nabelschnur bei Walen und Huftieren, mit besonderer
Berücksichtigung des europäischen Hausrindes. Acta Morphol.
Neerl.-Scand. 4:31-60, 1961.
Paglin,
D.E., Valentine, W.N., Miller, R.E., Nakatani, M. and Brockway, R.A.:
Acute intravascular hemolysis in the black rhinoceros: Erythrocyte enzymes
and metabolic intermediates. Amer. J. Vet. Res. 47:1321-1325, 1986.
Patton,
L., Czekala, N. and Lance, V.: Workshop on problems associated with the
low rate of reproduction among captive-born female Southern White Rhinoceros
(Ceratotherium simum simum). Zoological Society of San Diego, 1998.
Radcliffe,
R.W., Czekala, N.M. and Osofsky, S.A.: Technical Report. Combined serial
ultrasonography and fecal progestin analysis for reproductive evaluation
of the female white rhinoceros (Ceratotherium simum simum): Preliminary
results. Zoo Biol. 16:445-456, 1997.
Ramsey,
E.: The Placenta of Laboratory Animals and Man. Holt, Rinehart and Winston,
NY 1975.
Rüedi,
D. The great Indian rhinoceros. Chapter 18, pp. 171-190. In, One Medicine,
Ryder, O.A. and M.L. Byrd, eds. Springer-Verlag, New York, 1984.
Ryder,
O.A., ed.: Rhinoceros Biology and Conservation. Zoological Society of
San Diego, 1993 (Proc. of Conference, 1991).
Schaller,
K. and Pilaski, J.: Pocken bei Breitmaulnashörnern (Ceratotherium
s. simum) im Zoologischen Garten Münster. Zool. Garten 49:169-184,
1979.
Schwarzenberger,
F., Rietschel,W., Vahala, J., Holeckova, D., Thomas, P., Maltzan, J.,
Baumgartner, K. and Schaftenaar, W.: Fecal progesterone, estrogen, and
androgen metabolites for nonivasive monitoring of reproductive function
in the female Indian rhinoceros, Rhinoceros unicornis. Gen. Compar.
Endocrinol. 119:300-307, 2000.
Silberman, M.S. and Fulton, R.B.: Medical problems of captive and wild
rhinoceros - a review of the literature and personal experiences. J. Zoo
Anim. Med. 10:6-16, 1979.
Wurster-Hill, D.H. and Benirschke, K.: The chromosomes of the Great Indian
Rhinoceros (Rhinoceros unicornis L.). Experientia 24:511, 1968.
|