| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
Pelea capreolus
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
This single species Pelea comes from South Africa and must be differentiated from other species with similar appearances (Walther, 1968). It weighs around 20-30 kg. The aggressive males have short, pointed straight horns and the animals live in small family groups mostly in the hills and mountains (Nowak, 1999). The Boers applied the name “Rehbock” because of their apparent similarity in size and appearance to the deer with which they had been familiar. The longevity in zoos is 12 years and 4 months according to Jones (1993), in the wild, however, the longevity was given as being 8-10 years (Walther, 1968). The animals are “conservation-dependent” according to the IUCN.). They are rarely seen in zoos. Vrba & Schaller (2000) indicated that they first appeared in Africa around 3.7 MYA. They also suggested a phylogenetic relationship among the Pelelini and the saigas, rupicaprines, and antelopes etc. which derived later (see also Gatesy et al., 1997). Matthee & Robinson (1999) studied the relationship of numerous African bovidae with cytochrome b investigations and found the rhebok to be an outlier (see also Hassanin & Douzery, 1999).
 |
Rhebok at San Diego Zoo. |
2) General Gestational Data
The length of gestation is said to be 261 days (Brand, 1963; Nowak, 1999) with single calves born. Twins have been referred to as being born in November-December by Walther (1968). The weight of newborns has not been quoted. Breeding is seasonal with births occurring (in South Africa ) between September and December (Mentis, 1972). Dams have two mammae and no horns.
3) Implantation
There are no reports on early stages of pregnancy.
4) General Characterization of the Placenta
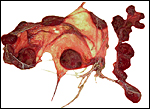
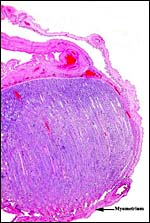
I have had one placenta from a successful birth at San Diego Zoo that weighed 265 g, measured 53 x 42 x 0.5 cm and possessed 21 cotyledons. Some of them were very small, as is seen in the photograph. Its cord was very short when received but the actual length is unknown. It is a cotyledonary, presumably syndesmochorial placenta. The larger cotyledons of this placenta must have been in the uterine horn with the fetus, the small ones are in the “empty” horn. Since then a pregnant female died in March, 2007 from pneumonia, approximately half-way through gestation. Its female fetus weighed 700g with a 29 cm CR length and 8 cm umbilical cord. The major portion of placenta and the fetus were in one horn and had 13 cotyledons (5 cm in greatest dimension) and 13 smaller cotyledons were in the unoccupied horn. Thick endocervical mucus was occluding the endocervical canal. In addition, a term fetus was born whose 238 g placenta shows much better preservation than was available before. This placenta had 15 cotyledons but a very torn umbilical cord that could not be measured.
5) Details of fetal/maternal barrier
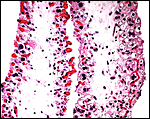
This placenta has an epithelio-chorial relationship. Binucleated trophoblastic cells are abundantly present. This placenta has the frequent “edema” of villi that is commonly associated with delivered placenta that have lain on the ground for some hours before keepers obtain the organ for study. The finer fetal vessels are prominently present beneath the trophoblast. There was no “hemophagous organ” in this placenta.
6) Umbilical cord
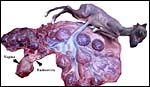
The length of the umbilical cord is not known to me; it possesses four large vessels and an allantoic duct. The immature fetus shown above had an 8 cm long cord with numerous ‘caruncles’.
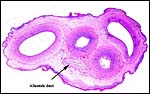 |
Cross section of immature umbilical cord. Red foci on the surface are areas of squamous metaplasia. |
7) Uteroplacental circulation
I am not aware of any studies.
8) Extraplacental membranes
These are unknown.
9) Trophoblast external to barrier
Judging from the implanted placenta shown and although I have not had an implanted placenta, judging from other related species, it is unlikely that there is trophoblastic infiltration into the uterus.
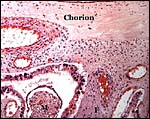
10) Endometrium
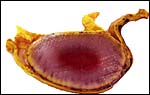
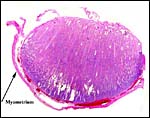
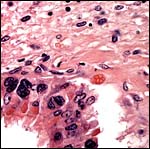
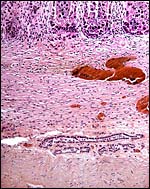
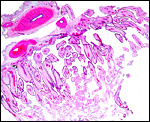
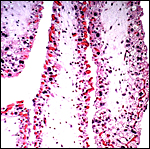
There is no decidualization, but the stroma between glands is fibrous and firm. This is shown at the implantation site shown above. The endocervix has thick mucus as shown next but what was interpreted as being vagina above, is lined by columnar epithelium as seen below.
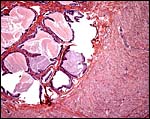 |
Endocervical canal is lined with thick mucus. |
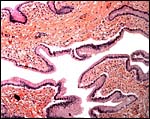 |
Columnar epithelium lines what appears to be the vagina. |
11) Various features
No unusual features are present.
12) Endocrinology
No studies can be found in the literature.
13) Genetics
Rheboks have 56 chromosomes according to findings made at CRES by Marlys Houck. An example of their unpublished findings is shown next. Hybrids have not been described.
 |
Placenta of a term gestation from a Rhebok. The cotyledons shown on the right are doubtless in the unoccupied uterine horn. |
14) Immunology
I am not aware of any studies.
15) Pathological features
A variety of parasites have been isolated from captive rheboks; thus, a Trichostrongyloid nematode Paracooperioides peleae (Boomker et al., 1981); Ostertagia triquetra (Boomker & Durette-Desset, 2003); Haemonchus contortus (Boomker et al., 1983); Haemonchus horaki (Lichtenfels et al. (2001); and Amblyomma marmoreum ticks (Horak et al., 1987; Horak & Boomker, 1998; Horak et al., 1982). No other reports of pathological findings are available.
16) Physiologic data
I am not aware of any studies.
17) Other resources
Cell strains from ear notches are available from CRES at San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu .
18) Other remarks – What additional Information is needed?
Early stages of implantation and features of the umbilical cord are needed.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society of San Diego. I am most grateful to Marlys Houck of CRES for allowing me to show the as yet unpublished karyotype of a rhebok.
References
Boomker, J. and Durette-Desset, M.C.: Parasites of South African wildlife. XVII. Ostertagia triquetra n.sp. (Nematoda:Trichostrongylina) from the grey rhebuck Pelea capreolus (Forster, 1790). Onderstepoort J. Vet. Res. 70:37-41, 2003.
Boomker, J., Horak, B. and de Vos, V.: Paracooperioides peleae gen. et sp. N. (Nematoda: Trichostrongylidae) from the vaal ribbok, Pelea capreolus (Forster, 1790). Onderstepoort J. Vet. Med. 48:169-174, 1981.
Boomker, J., Horak, I.G., Gibbons, L.M. and de Vos, V.: Haemonchus contortus from the vaal ribbok, Pelea capreolus , and the bontebok, Damaliscus dorcas dorcas , in the Bontebok National Park . Onderstepoort J. Vet. Res. 50:179-181, 1983.
Brand, D.J.: Records of mammals bred in the National Zoological Gardens of South Africa during the period 1908-1960. Proc. Zool. Soc. London 140:617-659, 1963.
Gatesy, J., Amato, G., Vrba, E., Schaller, G. and DeSalle, R.: A cladistic analysis of mitochondrial ribosomal DNA from the Bovidae. Mol. Phylogenet. Evol. 7:303-319, 1997.
Hassanin, A. and Douzery, E.J.: The tribal radiation of the family Bovidae (Artiodactyla) and the evolution of the mitochondrial cytochrome b gene. Mol. Phylogenet. Evol. 13:227-243, 1999.
Horak, I.G. and Boomker, J.: Parasites of domestic and wild animals in South Africa . XXXV. Ixodid ticks and bot fly larvae in the Bontebok National Park . Onderstepoort J. Vet. Res. 65:205-211, 1998.
Horak, I.G., de Vos, V. and de Klerk, B.D.: Helminth and arthropod parasites of vaal ribbok, Pelea capreolus , in the western Cape Province . Onderstepoort J. Vet. Res. 49:147-148, 1982.
Horak, I.G., MacIvor, K.M., Petney, T.N. and de Vos, V.: Some avian and mammalian hosts of Amblyomma hebraeum and Amblyomma marmoreum (Acari:Ixodidae). Onderstepoort J. Vet. Res. 54:397-403, 1987.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Lichtenfels, J.R., Pilitt, P.A., Gibbons, L.M. and Boomker, J.D.: Haemonchus horaki n. sp. (Nematoda: Trichostrongyloidea) from the grey rhebuck Pelea capreolus in South Africa . J. Parasitol. 87:1095-1103, 2001.
Matthee , C.A. and Robinson, T.J.: Cytochrome b phylogeny of the family Bovidae: Resolution within the alcelaphini, antilopini, neotragini, and tragelaphini. Mol. Phylogenet. Evol. 12:31-46, 1999.
Mentis, M.T.: A review of some life history features of the large herbivores of Africa . The Lammergeyer16:1-89, 1972.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Vrba, E.S. and Schaller, G.B.: Antelopes, Deer, and Relatives. Fossil record, behavioral ecology, systematics, and conservation. Yale University Press, New Haven , 2000.
Walther, F.: Kuhantilopen, Pferdebocke und Wasserbocke. Chapter 14, pp. 437-471, in Grzimek's Tierleben, Vol. 13, B. Grzimek, ed. Kindler Verlag, Zurich, 1968.