|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.) |
| Feb 2, 2008. |
Oryctolagus cuniculus
Order:
Lagomorpha
Family: Leporidae
1)
General zoological data of species
The domestic rabbit, often erroneously assumed to be synonymous with the
New Zealand White Rabbit, is widely used in research laboratories. There
are now, however, numerous other strains of domestic rabbit, and many
feral rabbits occur as well.
Rabbits belong to one of the two families of Lagomorpha (the Leporidae),
the other being the pikas (Ochotonidae). Their genetic relationship
was reviewed by Stock (1976) and by Dave et al. as well (1965). The domestic
rabbit is considered to have been derived from the wild rabbits of England,
Oryctolagus cuniculus. There are well over 50 "breeds"
of rabbits. They vary widely in size, genetic makeup, and in coloration
(Miller, 1999). Rabbits possess three (or four) upper incisor teeth, rather
than the two incisors found in rodents. Like the teeth in rodents, they
continue to grow throughout life in rabbits. Domestic rabbits weigh between
2 and 6 kg and have a life expectancy of about 6 years. Two comprehensive
reviews of the biology and diseases of rabbits have been published, one
by Kraus et al. (1984), and the other by Miller (1999).
Gotch (1979) described the origin of the term "oryctolagus"
as deriving from the Greek (orukter = tool for digging; lagos = a hare;
cuniculus = a rabbit).
 |
New Zealand White Rabbit. |
2)
General gestational data
The length of gestation in rabbits is 31-32 days; ovulation occurs about
10 hours after coitus, following a complex endocrine cascade.The rabbit
is thus a reflex ovulator (Fox & Laird, 1970). As many as 10 offspring
may be produced, more often there are 4-6. The weight at birth is 50-60
g. The average placental weight is 4 g (Mårtensson, 1984), and puberty
occurs at 4-6 months. The tubal transport of fertilized ova and blastocysts
was studied by Tsutsumi et al. (1975). They showed that "alien"
eggs catch up with the transport of normal eggs rapidly and they reached
the uterus within 6 hours. The rabbit has an "uterus duplex" (completely
separate horns) and the placental implantation is superficial, with inversion
of the yolk sac (Mossman, 1987). It develops a discoid, labyrinthine, chorio-allantoic
placenta, with a hemodichorial feto-maternal interface.
3) Implantation
Following the initial observations by Amoroso (1961), Miller (1999) showed
that a mucin coat is placed on the surface of the developing embryo as it
passes through the tubal isthmus. At the time of its initial implantation
(day 7-8 p.c.), the blastocyst is much enlarged, and the zona pellucida
has disappeared. Implantation of the symmetrical pair of "placental
folds" that exist on the blastocyst surface takes place antimesometrially.
Amoroso (1961) described the finer details of these early contacts and of
blastocyst invasion beautifully. There are two folds on the blastocyst surface,
not only at the antimesometrial side where the disks develop, but also on
the opposite side (the "ob-placental region"), where the yolks
sac contact with the endometrium takes place, with the subsequent development
of many multinucleated giant cells of trophoblastic origin. The definitive
placenta, however, develops mesometrially and it is essentially bilobed.
One prominent feature of rabbit implantation is the development of numerous
multinucleated giant cells in the endometrium. They reach their maximal
development by day 17 and, thereafter, they gradually disappear. These were
first interpreted as being of endometrial derivation, but later they were
considered to have a trophoblastic origin, which is also the current view.
Petry & Kühnel (1966) depicted these giant cells in the "chorion
laeve" as well. They suggested the possibility that these cells accomplish
much protein transport to the fetus. Because of the complexity of the ensuing
development, and because of the unusual nomenclature employed in descriptions
of rabbit placentation, two diagrams of Amoroso's (1961) excellent description
are shown next.
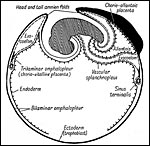 |
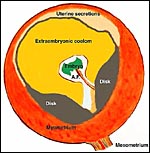
Figure 15.9 of Amoroso (1961) to illustrate the three parts of the rabbit yolk sac and the folding origin of the amnion. |
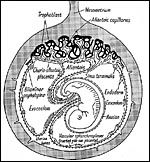 |
Figure 15.8 of Amoroso (1961) showing complete yolk sac inversion and anastomoses between yolk sac and allantoic blood vessels. Note the small (permanent) allantoic cavity and large exocoelom. |
The
rabbit placenta has, in addition to the bidiscoid chorioallantoic portion,
a completely inverted yolk sac as is shown above. Please also observe the
rather large exocoelomic space and the very small allantoic sac, especially
when the latter is compared with the massive size found in some other species.
This has suggested to some observers that waste products and fluids are
exchanged only through the placenta, rather than involving the allantois.
At this "ob-placental" pole of implantation there is much endometrial
proliferation and modification of its surface. While this yolk sac placentation
flourishes at first, it regresses later in gestation and it probably plays
then a less important site of exchange.
Since the initial phases of implantation are likely to be related to the
interactions and recognition of surface molecules, Biermann et al. (1997)
delineated the carbohydrate ligands of surface lectins of the Fallopian
tube, endometrium, and blastocysts in rabbits. Denker & Hafez (1975)
showed experimentally that, most likely, some trophoblastic enzymes rather
than endometrial factors initiate implantation of the blastocysts.
Implantation in rabbits thus begins on day 7, at the time when Tscheudschilsuren
et al. (1999) saw the first strong expression of an arylhydrocarbon receptor
and a translocator on the endometrial glandular surface. Grundker &
Kirchner (1996) studied the effect of growth factors on rabbit blastocysts.
Insulin-like growth factors (IGF-1 and IGF-2), as well as basic fibroblast
growth factor (FGF-2), were found in endometrial secretions. FGF-2 became
especially plentiful on day seven. IGF-1 was found to enhance blastocyst
expansion, while IGF-2 had no effect. FGF-2 caused the development of trophoblastic
knobs. Other growth factors were explored by Klonisch et al. (2001). They
studied EGF and TGF in the early interaction of rabbit blastocysts with
endometrium and found strong evidence that the "ErbB/EGF" system
plays a role in the peri-implantation period.
The actual discoid, chorio-allantoic placenta commences its development
on about the 12th day p.c., four days after blastocyst attachment and following
increased secretion by the uterine glands. A prominent feature of this development
is the degeneration of the swollen decidua. Amoroso (1961) described it
thus: "The uterine glands are, however, only temporary pathways
to the invasion of the trophoblast. They eventually disappear completely
and are replaced on the tenth day by multinucleate decidual cells. [It
should here be pointed out that the endometrial origin of these cells needs
reinvestigation; see Mossman, 1987, p. 216]. The dips thus correspond
to the gland orifices and represent the beginnings of so-called villi".
Amoroso stated that the endothelial barrier disappears completely by the
tenth day. Vascular mesoderm then invades the trophoblastic columns. These
are tubes that surround maternal blood channels, and the complexity of these
structures increases with advancing gestation considerably so as to forming
a typical placental labyrinth. Focal maternal hemorrhages occur at the depth
of the disks.
The rabbit trophoblast begins to tap maternal blood vessels on day 9, two
days after implantation has taken place (Hoffman et al., 1990). Thereafter,
the "labyrinthine hemodichorial, chorioallantoic placenta", with
inversion of the permanent yolk sac, develops (Enders & Blankenship,
1999). These authors drew attention to the very irregular thickness of regions
in the placenta, with outer syncytium placed over a layer of cytotrophoblast
and they diagrammed the hemal interface. As in rodents (see chapter on mouse),
antibodies and other proteins are transferred through the inverted yolk
sac region. The reader is also directed to the detailed description of rabbit
implantation by Hoffman et al. (1999).
Hafez (1964; 1965) superovulated rabbits and transferred an excessively
large number of blastocysts to does. While implantation often occurred in
the overcrowded uterine horns, there was much resorption after implantation
and fewer live pups were born than was the number of blastocysts that were
transplanted. Some of the neonates were growth-retarded, some had congenital
anomalies. Some placentas were fused because of crowding. Fujimoto et al.
(1975) enumerated the number of blastomeres at different days before implantation
and also studied their chromosomal complements. On day 4 there were 1,766
cells, on day 6 there were 98,790. The diameter of triploid blastocysts,
induced by delayed fertilization, is reduced (Shaver & Carr, 1969).
Moreover, triploid ova had a significant mortality.
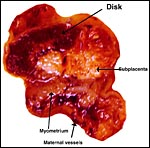
4) General characteristics of placenta
The rabbit placenta is discoidal when viewed from the fetal surface, but
Mossman (1987) pointed out that it is rather more bidiscoidal when sectioned
because of the development of a striking "groove" on the fetal
surface of the disk in the sagittal plane of the uterus. The bidiscoidal
structure is the result of the two surface folds visible already on the
blastocyst. There is late, complete inversion of the yolk sac and development
of a labyrinthine hemodichorial placental disk. The allantoic sac is relatively
small. At term, there is virtually no amnionic fluid and the amnion is extremely
thin and apposes the fetal skin directly.
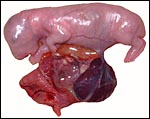
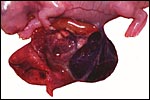
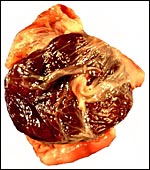
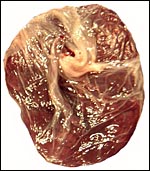
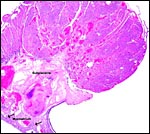
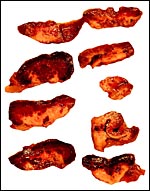
The specimens shown above were kindly donated by Dr. G. Heldt at UCSD. They come from a 27 day gestation of a strain that normally delivers on day 31. There were nine fetuses, each weighing between 30 and 34 g. The placentas, detached from the uterus, weighed between 85 and 88 g. The portion of uterus to which they were attached weighed 11 g. The doe was 4.5 kg.
5)
Details of barrier structure
The hemodichorial placental "membrane" of interchange in the
rabbit has alternating "thin and thick" regions (Enders &
Blankenship, 1999). Both display a layer of cytotrophoblast, above which
is syncytium. In some regions, however, the proximity of maternal and
fetal blood is very close and seemingly only separated by fetal capillary
endothelium. Amoroso (1961) suggested that, here, the trophoblast is deficient
and that, basically, a hemo-endothelial relation is formed. In addition,
there appear areas of numerous large, polyploid, multinucleated trophoblastic
cells in the endometrium. They are now considered to be fetal tissue and
to originate from the bilaminar omphalopleur (Mossman, 1987). These cells
completely surround the maternal arteries, invade them and, in time, they
even reach the myometrium within the maternal vessels. This deep penetration
of trophoblastic cells was commented upon in Mossman's last page of his
early vascular paper (1926) and will be of further concern in discussions
on rodent and guinea pig implantations.
Larsen (1961) has studied the implantation site electronmicroscopically.
The vacuoles that were present in the syncytium merged into lacunae that
become filled with maternal blood after invasion of her blood vessels,
coinciding with the time course provided by Hafez & Tsutsumi (1966).
Yolk sac attachment takes place on day 7 p.c., with relatively poor vascular
supply in that region.
6)
Umbilical cord
In contrast to rats, rabbit fetuses are said to have already separated from
their placenta and membranes at the time of birth (Hudson et al., 1999).
Miller (1999), on the other hand, stated that the dam severs the cord by
biting and that, thereafter, placentophagy occurs. Parturition is essentially
bloodless though, despite the abundance of maternal vessels in the pregnant
uterus.
There are two umbilical arteries that pursue a course beside the bladder
in the fetus and an umbilical vein passing to the liver. The cord is 2 cm
long and has no spirals.
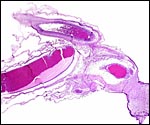 |
Cross section of umbilical cord. |
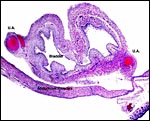 |
Fetal bladder with adjacent umbilical arteries. |
7)
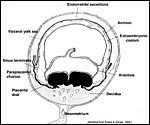
Uteroplacental circulation
The vascularization of the endometrium and of the placenta is complex; moreover,
it changes over time. The most comprehensive publication on the topic is
that by Mossman (1926). He described in great detail the changing vasculature
during development following a protocol of a detailed injection technique.
Particularly well studied were the arterial supply to the sinusoidal spaces
below the chorionic membrane, the infiltration of the maternal vessels by
giant cells, the sinus terminalis of the fetus, and the dissolution of much
of the large amount of decidua that develops beneath the fetal cotyledons.
His studies suggested that maternal blood is directed to the undersurface
of the chorionic membrane into the sinusoids; it then percolates towards
the maternal floor through trophoblastic "tubes", to be drained
by uterine veins. The fetal blood takes an opposite flow, thus establishing
countercurrent blood flows for maximal exchange possibilities. I show next
a diagram from Hafez & Tsutsumi (1966) that depicts this complex circulation
diagrammatically.
Nucleated red blood cells of the fetus disappeared around day 20-22. Mossman
was also much concerned with the large number of leukocytes present in early
rabbit implantation. In addition, he wrote detailed accounts of the trophoblastic
"tubes". Mossman then still considered that the rabbit placenta
was an endothelio-chorial organ. Later studies have disproved this. Hafez
& Tsutsumi (1966) have later also analyzed the blood vessels throughout
gestation and presented numerous other diagrams of the blood vessels beneath
the placenta and between the conceptuses. Their findings suggested that
critical vascular changes occur in rabbit gestation around the 20th day.
With advancing gestation, the number of endometrial vessels decreases, but
they increase in diameters. Other references to studies on uterine and placental
blood flows may be found in Mårtensson's publication (1984).
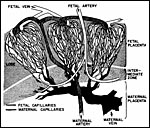 |
Diagram of the circulation in the placenta of rabbits. Minimally modified from Fig. 5 of Hafez & Tsutsumi, 1966. |
8)
Extraplacental membranes
The amnion is avascular, as in other species, and it is extremely thin.
No amnionic fluid was present in these placentas, as is usual at this late
gestation. The small allantoic sac is only slightly filled with fluid of
meso/metanephric origin but its composition changes over time (Krespi &
Davies, 1963). These authors have studied the nature, composition, origin
and disposition of the fetal fluids in several publications.
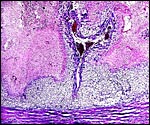
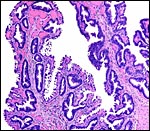 |
Yolk sac placental surface with columnar epithelium. |
9)
Trophoblast external to barrier
There is invasion into the decidua and deep into the myometrium by the giant
trophoblastic cells (see Mossman, 1926). These giant cells are also found
within maternal blood vessels walls, within the lumens and also in veins.
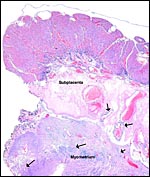
The infiltration is especially prominent in the "subplacental"
vessels (next photo). But when deeper levels are studied, within the myometrium
the dark color of cells disappears, and they are no longer multinucleate
or "giant". They are still endowed with purple cytoplasm and infiltrate
to near the uterine serosal surface among muscle bundles. I doubt, however,
that they are the same trophoblastic cells; but they are confined to the
placental site, not seen next to it, and they are not decidual cells. As
is indicated above, future studies need to be directed towards the elucidation
of this multitude of specialized cells. They are particularly puzzling in
hystricomorph rodents where they are a much larger population of diverse
cells.
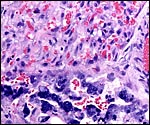
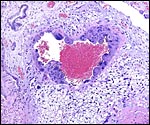 |
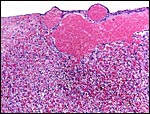
Maternal blood vessel within the subplacenta is lined by trophoblastic giant cells. Vacuolated endometrial stroma (right and below) and endometrial gland (top left). |
10)
Endometrium
In contrast to ungulates, the endometrium beneath the implanting placental
disk has endometrial glands (Amoroso, 1961). Mossman (1987) described the
manner of the abundant decidualization at the base of the rabbit placenta.
He contended that the rabbit placenta separates at delivery in a decidual
plane, the likes of which are "never more pronounced", he stated.
The uterine surface epithelium between the implantation sites enlarges markedly
and has the appearance of a secretory tissue. Gray et al. (2001) reviewed
the manner of uterine secretion (embryotrophe) of various species, and Miller
(1999) reviewed the endometrial changes during implantation.
Mossman (1987) depicted the large, multinucleated giant epithelial cells
in the surface of the rabbit endometrium (also in the nonpregnant animal)
and concluded that their multiple nuclei or enlarged nuclei result from
endomitosis. In his earlier contribution (1926), he showed these giant cells
as surrounding the arterial walls of the deeper layer of the implantation
site and thence their infiltration into the arterial lumens. There has been
much controversy as to the origin of these giant cells, some believing them
to derive from decidua, other derive them from trophoblast. Largely because
of their lack of glycogen (a characteristic for decidua), Amoroso (1961)
firmly decided for a trophoblast derivation.
11)
Various features
Mossman (1987) emphasized in his book on comparative placentation that,
in the subplacenta, beneath the groove that develops on the fetal surface
of the disk, remnants of uterine epithelium are found. ... "and
a uterine cavity of what was the depth of the sulcus between the two mesometrial
endometrial folds" (after Mossman, 1926).
12)
Endocrinology
Miller (1999) presented a summary of the endocrine parameters of rabbit
gestation. Although rabbits are reflex ovulators, ovulation can be induced
by hCG. The mechanism by which the corpus luteum is maintained during
pregnancy is still poorly understood. Current studies suggest that a mitochondrial
protein (StAR) is involved (Miller, 1999). The placenta produces relaxin
in its syncytiotrophoblast, but no progesterone is secreted. Hafez (1964)
showed that progesterone administration to oophorectomized rabbits maintains
the pregnancy. There is also no estrogen production by the rabbit placenta
(Mårtensson, 1984). Superovulation with hCG is successful and produces
large numbers of blastocysts, often of an increased size (Hafez &
Rajakoski, 1968).
In order to understand the possibly adverse effect of glucocorticoids
upon fetal development, Hundertmark et al. (2001) studied the localization
of 11beta-hydroxysteroid dehydrogenase activity. The enzyme was found
in the placenta, colon and kidney of fetal rabbits. The expression of
17beta-hydroxysteroid dehydrogenase was examined by Krusche et al. (2001).
They detected it in the placenta, and also in other tissues. Bouraima
et al. (2001) examined aromatase-encoding genes and identified promoter-derived
transcripts in ovary, fat, and placenta.
The finding that there is no appreciable stimulation of the interstitial
compartment of the fetal testes suggests to me that there is little fetal
gonadotropin in the fetal circulation.
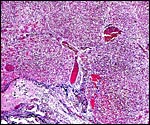
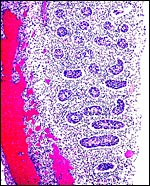 |
Fetal testis at 27 days of gestation. Interstitial cells are not stimulated. |
13)
Genetics
The rabbit has 44 chromosomes, as is attested to by numerous publications (Nichols et al., 1965; Ray & Williams, 1966; Issa et al., 1968; Hsu & Benirschke, 1967). It thus differs from hares and other Lagomorpha which generally have higher chromosome numbers (Dave et al., 1965; Stock, 1976). Hageltorn & Gustavsson (1979) published the findings of chromosome banding studies. The "sex chromatin" or “Barr body” is evident in fibroblasts (Melander, 1962; Hulliger et al., 1963), and early sex determination of blastocysts was thus accomplished by Edwards & Gardner (1967). Initial gene assignments have been reported by Soulié & de Grouchy (1982, 1983). Martin & Shaver (1979) reported on a fertile male rabbit with an extremely small Y-chromosome. Most recently, Korstanje et al. (2003) have established linkages to certain chromosomes with microsatellite markers.(See below).
 |
Karyotypes of male and female domestic rabbits. |
Spontaneous hybrids between the domestic rabbit and other leporids have
not been described. Chang et al. (1964), however, found that artificial
insemination of rabbits with semen of the snowshoe hare (Lepus americanus)
yielded some fertilized ova, but almost all eventually degenerated before
implantation. Only one such hybrid implanted and developed a small embryo.
When snowshoe hares were inseminated with rabbit semen and pretreated with
hCG, 90% were fertilized and two young were produced (Chang, 1965). Blastocyst
transfer failed to induce a normal endometrial response.
Fujimoto et al. (1975) examined the number of blastomeres and chromosomes
of superovulated, fertilized rabbit ova. Approximately 9% of these ova were
chromosomally abnormal, including exhibiting triploidy. Triploidy can also
be induced by delayed fertilization and it is mostly the result of digyny
(Shaver & Carr, 1969; see also Austin, 1967). Milde et al. (2001) studied
a "proteolipid protein 2 mRNA" expression in embryos. They found
this motif, that is similar to PP2/A4 of man, mouse and sheep, to be expressed
at the posterior pole of the gastrulating embryo on day 7.
Numerous hereditary diseases have been described in domestic rabbits. They
are summarized by Kraus et al. (1984). Omphalocele and gastroschisis are
not uncommon, according to Heldt (G. P. Heldt, UC San Diego, Personal communication).
The remarkable overpopulation of rabbits in Australia is legendary. Cooper
& Herbert (2001) have recently reviewed its consequences. As few as
13 animals are said to have been the stock from which this expansive population
derived. Added to this, in 1950, the myxomatosis virus was intentionally
introduced in Australia, with massive deaths but with subsequent gradual
resurgence of a resistant strain and modification of the virus. Similarly,
calicivirus infection has been unable to significantly reduce the rabbit
population in Australia, owing probably to mutations, selection, and transplacental
antibody exposure.
"The genetic map of the rabbit is underdeveloped…"stated
Korstanje et al. (2001) in a paper on the analysis and mapping of various
biochemical markers in two inbred strains of rabbit. They found some polymorphisms,
and none in some other systems. The paper provides access to modern genetic
studies of rabbit genotypes.
14)
Immunology
Early studies of the rabbit placentation were designed to understand the
transport of immunoglobulins from mother to fetus (Mossman, 1926). It
has since become clear that much of this transport is the result of the
inverted yolk sac activity. Because of their gestational characteristics,
rabbits have also been used to better understand possible modes for gene
therapy (Heikkila et al., 2001). Intravascular, guided catheters were
employed for the attempted infection with various gene constructs. No
placentitis occurred; adenovirus constructs were the most infective.
Korstanje et al. (2001) studied various immune and serologic markers in
inbred strains of rabbits.
15)
Pathological features
Two comprehensive reviews of diseases of rabbits have been published,
one by Palmer (1978), and the other by Kraus et al. (1984). Most notorious
of leporid diseases probably is the nearly 100% fatal myxomatosis. The
virus responsible for this infection was introduced into the Australian
population in 1950. This was undertaken in an effort to eliminate the
rabbit pest experienced in that country (Cooper & Herbert, 2001).
Rabbit hemorrhagic disease caused a significant mortality in adult rabbits
in Spain (Villafuerte et al., 1994). Calicivirus infection is another
serious illness of rabbits. The rabbit has also frequently served as a
model for teratogenesis, and for in utero infections. Thus, Qian
et al. (2000) showed convincingly that Schistosoma japonicum can
be transmitted transplacentally. Likewise, Cere et al. (2000) affirmed
that Pneumocystis can be acquired in utero. This organism
is the cause of a nearly ubiquitous infection of adult rabbits. Davies
et al. (2000) showed that fetal/placental infection occurs when E.
coli organisms were placed endocervically during pregnancy. Leslie
et al. (2000) followed the cytokine responses of pregnant rabbits that
were infected in utero in the third trimester. Likewise, Gibbs
et al. (2002) found that rabbit placentas and fetuses can become infected
by intracervical injection of E. coli and that antibiotic therapy
does not completely eradicate the fetal infection. Rabbits can also become
infected, and they are ill after transmission, by the malignant catarrhal
fever virus (Buxton & Reid, 1980). Infection with toxoplasma and encephalitozoon
was shown by Waller & Bergquist (1982).
Experiments conducted by Kato et al. (2001) with granulocyte colony-stimulating
factor showed thrombosis of placental vessels, with necrosis and abortion
following. Different results were obtained in rats. Zook et al. (2001)
studied the effect of estradiol and levonorgestrel administration to rabbits.
They produced decidualization and decidual tumors. These lesions were
not confined to female rabbits but occurred (in the spleens) of adult
male rabbits as well.
Henderson (1954) was interested in resolving the question of absorption
of fetal antigens into the pregnant doe and studied the possible continued
placental growth after fetal demise. The latter had been claimed to occur
in rhesus monkey pregnancies after fetal demise, but this was later disproved.
She caused fetal demise surgically in rabbits or, more rapidly, by stilbestrol
administration. Placental growth stopped and, so long as pregnancy continued
with some live pups, the uterus did not contract. Leukocytic infiltration
ensued and antigen transfer may have occurred after fetal demise.
Numerous genetic errors exist in the many different breeds of rabbit.
Some of these errors were summarized by Fox (1975). Palmer (1978) pointed
to the large percentage of spontaneous intrauterine resorptions and cautioned
that this feature needs to be known when teratogenic studies are conducted,
as experimental results may otherwise be misinterpreted.
Kaufmann-Bart & Fischer (2008) have reported a first case of chorocarcinoma in a domestica rabbit with lung metastases. Remarkably, the syncytial cells were immunopositive for anti-human hcg antibody.
16) Physiological data
Miller (1999) has superbly summarized much of what is known about rabbit
physiology, reproduction, and endocrinology (see also Kraus et al., 1984).
Hudson et al. (1999) observed rabbit and rat parturitions by videography.
They found rabbit births to occur much more rapidly than those of rats,
and stated that the pups are born already separated from the placenta.
This is contrary to the statements by Miller (1999) who described maternal
biting of the cord.
Papadopulos et al. (1999) developed fetoscopy in the rabbit model and
showed that successful fetoscopic evaluations can be carried out in the
rabbit, beginning with the second trimester.
In contrast to so many other mammalian species in which the ovary of newborns
has oocytes that have completed their first meiotic division, in rabbits,
this maturation occurs mostly postnatally (Teplitz & Ohno, 1963; Kennelly
& Foote, 1966). The process of oogenesis peaks on postnatal days 12-14
and is completed on neonatal day 20.
Hagey et al. (1998) have used bile acid structural modifications that
have taken place in evolution so as to ascertain disputed relationships
among mammals and birds. They showed that rabbits have an unique bile
acid form and discussed in this publication the possibility that either
the notorious coprophagy of rabbits or their enormous cecum may have contributed
to this feature. Glenister (1961) studied trophoblastic maturation of
rabbits in organ culture.
17)
Other resources
I am indebted to Dr. G. Heldt (UCSD) for these placentas. Cell strains
of rabbits and of several Sylvilagus species and of Lepus californicus
are available from CRES
by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other relevant features
Rabbits have been used for transporting sheep and cattle blastocysts over
long distances. Thus, for instance, Sreean & Scanlon (1968) showed
that blastocyst maturation and cleavage of eggs continued when cattle
blastocysts were placed into the Fallopian tubes and uteri of pseudopregnant
rabbits.
Mossman (1987) suggested that better studies are needed to definitively
identify the origin and physiology of the multinucleated giant cells of
the rabbit placenta. I believe that the uninucleate cells in the myometrium
require study and clarification.
References
Amoroso, E.C.: Placentation. Chapter 15, pp. 127-311, in Marshall's Physiology
of Reproduction, A.S. Parks, ed. Vol. II, Little Brown & Co., Boston,
1961.
Austin, C.R.: Chromosome deterioration in ageing eggs of the rabbit. Nature 213:1018-1019, 1967.
Benirschke, K. and Kaufmann, P.: The Pathology of the Human Placenta. 4th edition. Springer-Verlag, NY, 2000.
Biermann, L., Gabius, H.J. and Denker, H.W.: Neoglycoprotein-binding sites (endogenous lectins) in the Fallopian tube, uterus and blastocyst of the rabbit during the preimplantation phase and implantation. Acta Anat. 160:159-171, 1997.
Bouraima, H., Hanoux, V., Mittre, H., Feral, C., Benhaim, A. and Leymarie, P.: Expression of the rabbit cytochrome P450 aromatase encoding gene uses alternative tissue-specific promoters. Eur. J. Biochem. 268:4506-4512, 2001.
Buxton, D. and Reid, H.W.: Transmission of malignant catarrhal fever to rabbits. Vet. Rec. 106:243-245, 1980.
Cere, N., Drout-Viard, F., Dei-Cas, E., Chanteloup, N. and Coudert, P.: In utero transmission of Pneumocystis carinii sp. F. oryctolagi. Parasite 4:325-330, 1997.
Chang, M.C.: Artificial insemination of snowshoe hares (Lepus americanus) and transfer of their fertilized eggs to the rabbit (Oryctolagus cuniculus). J. Reprod. Fertil. 10:447-449, 1965.
Chang, M.C., Marston, J.H. and Hunt, D.M.: Reciprocal fertilization between the domestic rabbit and the snowshoe hare with special reference to insemination of rabbits with an equal number of hare and rabbit spermatozoa. J. Exp. Zool. 155:437-446, 1964.
Cooper, D.W. and Herbert, C.A.: Genetics, biotechnology and population management of over-abundant mammalian wildlife in Australia. Reprod. Fertil. Devel. 13:451-458, 2001.
Dave, M.J., Takagi, N., Oishi, H. and Kikuchi, Y.: Chromosome studies on the hare and the rabbit. Proc. Japan Academy 41:244-248, 1965.
Davies, J.K., Shikes, R.H., Sze, C.I., Leslie, K.K., McDuffie, R.S., Romero, R. and Gibbs, R.S.: Histologic inflammation in the maternal and fetal compartments in a rabbit model of acute intra-amniotic infection. Amer. J. Obstet. Gynecol. 183:1088-1093, 2000.
Denker, H.W. and Hafez, E.S.E.: Proteases and implantation in the rabbit: role of trophoblast vs. uterine secretion. Cytobiologie 11:101-109, 1975.
Edwards, R.G. and Gardner, R.L.: Sexing of live rabbit blastocysts. Nature 214:576-577, 1967.
Enders, A.C. and Blankenship, T.N.: Comparative placental structure. Advanced Drug Deliv. Rev. 38:3-15, 1999.
Fox, R.R.: The Rabbit, Oryctolagus cuniculus. Chapter 14, in Handbook of Genetics, Vol. 4. R.C. King, ed. Plenum Publ., N.Y. 1975.
Fox, R.R. and Laird, C.W.: Sexual cycles. In Reproduction and Breeding Techniques for Laboratory Animals. E.S.E. Hafez, ed. pp. 107-122. Lea & Febiger, Philadelphia, 1970.
Fujimoto, S., Pahlavan, N., Woody, H.D. and Dukelow, W.R.: Cell numbers in rabbit pre-implantation blastocysts. Cytologia 40:307-311, 1975.
Gibbs, R.S., Davies, J.K., McDuffie, R.S., Leslie, K.., Sherman, M.P., Centretto, C.A. and Wolf, D.M.: Chronic intrauterine infection and inflammation in the preterm rabbit, despite antibiotic therapy. Amer. J. Obstet. Gynecol. 186:234-239, 2002.
Glenister, T.W.: Observations on the behaviour in organ culture of rabbit trophoblast from implanting blastocysts and early placentae. J. Anat 95:474-484, 1961.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray, A.P.: Mammalian Hybrids. Second edition. A Check-List with Bibliography. Commonwealth Agricultural Bureaux, Farnham Royal, Slough, UK, 1972.
Gray, C.A., Bartol, F.F., Tarleton, B.J., Wiley, A.A., Johnson, G.A., Bazer, F.W. and Spencer, T.E.: Developmental biology of uterine glands. Biol. Reprod. 65:1311-1323, 2001.
Grundker, C. and Kirchner, C.: Influence of uterine growth factors on blastocyst expansion and trophoblast knob formation in the rabbit. Early Pregnancy 2:264-270, 1996.
Hafez, E.S.E.: Growth and survival of blastocysts in the domestic rabbit. II. Quantitative effects of exogenous progesterone following ovariectomy. J. Reprod. Fertil. 7:241-249, 1964.
Hafez, E.S.E.: Maternal effect on implantation and related phenomena in the rabbit. Experientia 21:234, 1965.
Hafez, E.S.E.: Effects of over-crowding in utero on implantation and fetal development in the rabbit. J. Exp. Zool. 156:269-288, 1966.
Hafez, E.S.E. and Rajakoski, E.: Growth and survival of blastocysts in the domestic rabbit. I. Effect of maternal factors. J. Reprod. Fertil. 7:229-240, 1964.
Hafez, E.S.E. and Tsutsumi, Y.: Changes in endometrial vascularity during implantation and pregnancy in the rabbit. Amer. J. Anat. 118:249-282, 1966.
Hageltorn, M. and Gustavsson, I.: Identification by banding techniques of the chromosomes of the domestic rabbit (Oryctolagus cuniculus L.). Hereditas, 90:269-279, 1979.
Hagey, L.R., Schteingart, C.D., Rossi, S.S, Ton-Nu, H-T. and Hofman, A.F.: An N-acyl glycyltaurine conjugate of deoxycholic acid in the biliary bile acids of the rabbit. J. Lipid Res. 3:2119-2124, 1998.
Heikkila,
A., Hiltunen, M.O., Turunen, M.P., Keski-Nisula, L., Turunen, A.M., Rasanen,
H., Rissanen, T.T., Kosma, V.M., Manninen, H., Heinonen, S. and Yla-Herttuala,
S.: Angiographically guided utero-placental gene transfer in rabbits with
adenoviruses, plasmid/liposomes and plasmid/polyethyleneimine complexes.
Gene Ther. 8:784-788, 2001.
Henderson, M.: Foetal regression in rabbits; experimental studies of histolysis
and phagocytosis. Proc. Royal Soc. Series B. # 906. 142:88-112, 1954.
Hoffman, L.H., Winfrey, V.P. and Hoos, P.C.: Sites of endometrial vascular leakage during implantation in the rabbit. Anat. Rec. 227:47-61, 1990.
Hoffman, L.H., Breinan, D.R. and Blaeuer, G.L.: The rabbit as a model for implantation: In vivo and in vitro studies. In, Embryo Implantation: Molecular, Cellular, and Clinical Aspects, D.D. Carson, ed. Springer-Verlag, NY, 1999.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes, Vol. 1, Folio 8, 1967. Springer-Verlag, NY.
Hudson, R., Cruz, Y., Lucio, A., Ninomiya, J. and Martinez-Gomez, M.: Temporal and behavioral patterning of parturition in rabbits and rats. Physiol. Behav. 66:599-604, 1999.
Hulliger, L., Klinger, H.P. and Allgöwer, M.: Sex chromatin as a marker in some rabbit cells. Experientia 19:240-, 1963.
Hundertmark, S., Buhler, H., Fromm, M., Kruner-Gareis, B., Kruner, M., Ragosche, V., Kuhlmann, K. and Seckl, J.R.: Ontogeny of 11beta-hydroxysteroid dehydrogenase: activity in the placenta, kidney, colon of fetal rats and rabbits. Horm. Metab. Res. 33:78-83, 2001.
Issa, M., Atherton, G.W. and Blank, C.E.: The chromosomes of the domestic rabbit, Oryctolagus cuniculus. Cytogenetics 7:361-375, 1968.
Kato, Y., Kuwabara, T., Itoh, T., Hiura, M., Hatori, A., Shigematsu, A. and Hara, T.: A possible relationship between abortions and placental embolism in pregnant rabbits given human granulocyte colony-stimulating factor. J. Toxicol. Sci. 26:39-50, 2001.
Kaufmann-Bart, M. and Fischer, I.: Choriocarcinoma with metastasis in a rabbit (Oryctolagus cuniculi). Vet. Pathol. 45:77-79, 2008.
Kennelly, J.J. and Foote, R.H.: Oocytogenesis in rabbits. The role of neogenesis in the formation of the definitive ova and the stability of oocyte DNA measured with tritiated thymidine. Amer. J. Anat. 118:573-590, 1966.
Klonisch,
T., Wolf, P., Hombach-Klonisch, S., Vogt, S., Kuechenhoff, A., Tetens,
F. and Fischer, B.: Epidermal growth factor-like ligands and erbB genes
in the peri-implantation rabbit uterus and blastocyst. Biol. Reprod. 64:1835-1844,
2001.
Korstanje, R., den Bieman, M., Campos, P.J., Esteves, P.J., Lankhorst,
AE., van der Loo, W., van Zutphen, L.F.M., van Lith, H.A. and Ferrand,
N.: Genetic analysis and mapping of biochemical markers in an F2 intercross
of two inbred strains of the rabbit (Oryctolagus cuniculus). Biochem.
Genet. 39:159-178, 2001.
Korstanje, R., Gillissen, G.F., Versteeg, S.A., van Ost, B.A., Bosma, A.A., Rogel-Gaillard, C., van Zutphen, L.F.M. and van Lith, H.A.: Mapping of rabbit microsatellite markers using chromosome-specific libraries. J. Hered. 94:161-169, 2003.
Kraus, A.L., Weisbroth, S.H., Flatt, R.E. and Brewer, N.: Biology and diseases of rabbits. Chapter 8, in: Laboratory Animal Medicine, J.G. Fox, B.J. Cohen and Loew, F.M., eds. Academic Press, 1984.
Krespi, V. and Davies, J.: Electrical potential differences across the foetal membranes of the rabbit. J. Embryol. Exp. Morphol. 11:167-174 1963.
Krusche, C.A., Moller, G., Beier, H.M. and Adamski, J.: Expression and regulation of 17 beta-hydroxysteroid dehydrogenase 7 in the rabbit. Mol. Cell Endocrinol. 171:169-177, 2001.
Larsen, J.F.: Electron microscopy of the implantation site in the rabbit. Amer. J. Anat. 109:319-334, 1961.
Larsen, J.F.: Electron microscopy of the chorioallantoic placenta of the rabbit. I. The placental labyrinth and the multinucleated giant cells of the intermediate zone. Ultrastructure Res. 7:535-549 1962.
Larsen, J.F.: Electron microscopy of the chorioallantoic placenta of the rabbit. II. The decidua and the maternal vessels. J. Ultrastructure Res. 8:327-38, 1963.
Leslie, K.K., Lee, S.L., Woodcock, S.M., Davies, J.K., McDuffie, R.S., Hirsch, E., Sherman, M.P., Eskens, J.L. and Gibbs, R.S.: Acute intrauterine infection results in an imbalance between pro- and anti-inflammatory cytokines in the pregnant rabbit. Amer. J. Reprod. Immunol. 43:305-311, 2000.
Mårtensson, L.: The pregnant rabbit, guinea pig, sheep and rhesus monkey as models in reproductive physiology. Europ. J. Obstet. Gynecol. 18:169-182, 1984.
Martin, P.A. and Shaver, E.L.: A fertile male rabbit with minute Y chromosome. J. Exp. Zool. 181:87-98, 1972.
Melander, Y.: Chromosomal behaviour during the origin of sex chromatin in the rabbit. Hereditas 48:645-661, 1962.
Milde, S., Viebahn, C. and Kirchner, C.: Proteolipid protein 2mRNA is expressed in the rabbit embryo during gastrulation. Mech. Devel. 106:129-132, 2001.
Miller, J.B.: Rabbits. In, Encyclopedia of Reproduction, Vol. IV. E. Knobil and J.D, Neill, eds., Academic Press, San Diego 1999.
Mossman, H.W.: The rabbit placenta and the problem of placental transmission. Amer. J. Anat. 37:433-497, 1926.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nichols, W.W., Levan, A., Hansen-Melander, E. and Melander, Y.: The idiogram of the rabbit. Hereditas 53:63-76, 1963.
Nowak,
R.M. and Paradiso, J.L.: Walker's Mammals of the World, Vol. II. 4th edition.
The Johns Hopkins University Press, Baltimore, 1983.
Palmer, A.K.: Rabbits. In Chapter 20 - Developmental Abnormalities, in:
Pathology of Laboratory Animals, Volume II, K. Benirschke, F.M. Garner
and T.C. Jones, eds. Springer-Verlag, N.Y., 1978.
Papadopulos, N.A., Dumitrascu, I., Ordonez, J.L., Decaluwe, H., Lerut, T.E., Barki, G. and Deprest, J.A.: Fetoscopy in the pregnant rabbit at midgestation. Fetal Diagn. Ther. 14:118-121, 1999.
Petry, G. and Kühnel, W.: Riesenzellen im Chorion laeve des Kaninchens. Experientia 22:443-444, 1966.
Ray, M. and Williams, T.W.: Karyotype of rabbit chromosomes from leucocyte cultures. Canad. J. Genet. Cytol. 8:393-397, 1966.
Qian, B.Z., Bogh, H.O., Johansen, M.V. and Wang, P.P.: Congenital transmission of Schistosoma japonicum in the rabbit. J. Helminthol. 74:267-270, 2000.
Shaver, E.L. and Carr, D.H.: The chromosome complement of rabbit blastocysts in relation to the time of mating and ovulation. Canad. J. Genet. Cytol. 11:287-293, 1969.
Soulié, J. and deGrouchy, J.: Of rabbit and man: Comparative gene mapping. Hum. Genet. 60:172-175, 1982.
Soulié, J. and deGrouchy, J.: New gene assignments in the rabbit (Oryctolagus cuniculus). Comparison with other species. Hum. Genet. 63:48-52, 1983.
Sreean, J. and Scanlon, P.: Continued cleavage of fertilized bovine ova in the rabbit. Nature 217:867, 1968.
Stock, A.D.: Chromosome banding pattern relationships of hares, rabbits, and pikas (order Lagomorpha). A phyletic interpretation. Cytogenet. Cell Genet. 17:78-88, 1976.
Teplitz, R. and Ohno, S.: Postnatal induction of ovogenesis in the rabbit (Oryctolagus cuniculus). Exp. Cell Res. 31:183-189, 1963.
Tscheudschilsuren, G., Hombach-Klonisch, S., Kuchenhoff, A., Fischer, B. and Klonisch, T.: Expression of the arylhydrocarbon receptor and the arylhydrocarbon receptor nuclear translocator during early gestation in the rabbit uterus. Toxicol. Appl. Pharmacol. 160:231-237, 1999.
Tsutsumi, Y., Oguri, N. and Hafez, E.S.: Rapid transport of alien eggs transplanted 66 hours post coitum in the oviduct of the rabbit. J. Reprod. Med. 14:62-63, 1975.
Villafuerte, R., Calvette, C., Gortazar, C. and Moreno, S.: First epizootic of rabbit hemorrhagic disease in free living populations of Oryctolagus cuniculus at Donna National Park, Spain. J. Wildl. Dis. 30:176-179, 1994.
Waller, T. and Bergquist, N.R.: Rapid simultaneous diagnosis of toxoplasmosis and encephalitozoonosis in rabbits by carbon immunoassay. Lab. Anim. Sci. 32:515-517, 1982.
Zook, B.C., Janne, O.A., Abraham, A.A. and Nash, H.A.: The development and regression of deciduosarcomas and other lesions caused by estrogens and progestins in rabbits. Toxicol. Pathol. 29:411-416, 2001.