| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: Feb 15, 2008 |
Pygmy Slow Loris
Nycticebus pygmaeus
Order: Primates
Family: Lorisidae
1) General Zoological Data
Of these Prosimians there are two slow loris species, N. coucang and N. pygmaeus; the others are N. intermedius and N. bengalensis (see Groves, 2001; Roos, 2004). The ‘pygmy’ species that is here considered comes from Vietnam. The placenta was kindly donated by Dr. Tilo Nadler of the EPRC in Cuc Phuong, Vietnam. It was bred at that location. This nocturnal animal has of course very large eyes and is arboreal in its normal habitat. It is insectivorous but also eats fruit, small mammals, lizards etc.; it is thus essentially omnivorous. These animals are relatively easily kept and bred in captivity and also appear often as pets and on Asiatic markets. The IUCN considers this species as being ‘vulnerable’ and Dr. Helena Fitch-Snyder of the San Diego Zoo is the studbook coordinator (Helena@sandiegozoo.org). She has written a detailed web site as well as a Loris Husbandry Manual.
 |
Pygmy slow loris with young at the San Diego Zoo. The thin dark stripe on the back is not visible. |
2) General Gestational Data
The maximum age for pygmy lorises was listed as being between 16 and 17 years by Weigl (2005). Gestation is said to be 185-192 days long (Nowak, 1999; Jurke et al. 1997 suggested 187-198 days from estrone determinations) and usually singletons are born. Twins, however, may occur as often as in slightly less than one-half of the gestations. The neonates weigh ~30-60 g while adults weigh 300-450 g, the males being slightly larger (Kappeler, 1991).
3) Implantation
Early implantational studies are not available for this particular species but are badly needed. They were detailed for its close relative, Nycticebus lydekkerianus (=tardigradus) by Hill et al. (1928) who observed three early embryos. It is likely that pygmy lorises have a very similar early development.
4) General Characterization of the Placenta
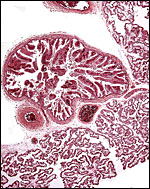
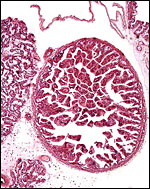
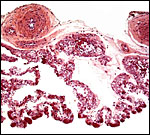
This placenta measured 3.5 x 2 x 0.8 cm in size, was discoid in shape, and it weighed only 4.3 g. While it is quite unusual to have an opportunity to study such a placenta, it was only marginally preserved and was also heavily contaminated by dirt. Moreover, the orientation of the surface, the insertions of the cord and membranes were impossible to ascertain upon arrival in the laboratory.
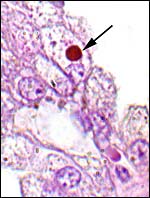
In a comprehensive literature search it appears that the placenta of the pygmy loris has not ever been studied. Mossman (1987) suggested that it must be similar to other Lorisidae (such as the slender Loris and Galago). Those placentas have been observed, especially that of the galago, and they are referred to by Mossman. He described them to be typical of the Strepsirhini that are represented by having a diffuse villous epitheliochorial type of placenta. That is to say that they have a diffuse microvillous structure, are surrounded by a “ring of allantochorion”, have ‘areolae’, their nidation is superficial, they have a temporary juxtauterine omphalopleure and a large, lobed allantois. The areolae of Loris lydekkerianus (see also Hill et al., 1928, and according to Enders [personal communication, 2008], this is a picture of the placenta from the slender loris, L. tardigradus – This specimen was also further described on p. S13 by Enders & Carter, 2006) were depicted by Mossman (1987) as being located and being contiguous with the ‘uterine milk’-producing endometrial glands, although they were then shown to contain much more secretion than is the case in my specimen. Moreover, since mine was a delivered placenta without attachment to the uterus, the identification of the localization of these areolae is tentative. They were always round structures without openings to the intervillous space (at least in these sections), and they were surrounded by a thin layer of smooth musculature that contained occasional small blood vessels (Enders & Carter, 2006 have shown similar features in color photographs on p. S13). A small amount of debris was found within them. The epithelial cells of the cristae stained much darker than those of the villous trophoblast, contained fine cytoplasmic vacuoles but only little stainable iron was found as is described by others to occur in pig placentas. In contrast, their cytoplasm had numerous globular protein inclusions that were usually located beneath the nuclei and were adjacent to the rich capillary bed. They are depicted below but were not present in the photographs shown by Enders & Carter (2006); an occasional globular inclusion had a deep yellow-brown color, which contained some iron moiety. Importantly, the epithelium of the cristae had a much lesser staining reaction with pan-cytokeratin preparations than the trophoblast of villi, while the wall of the areolae was deeply stained. Perhaps the epithelium has a different origin than trophoblast? Consider this possibility: might they not be ‘herniations’ into trophoblastic epithelium (their walls are deeply stained) into which endometrial epithelium grew? Only new material from attached placentas, especially young ones, and employing similar staining reactions will settle this point. Although they are commonly called ‘chorionic vesicles’, that may not be the proper appellation.
I found 11 such areolae in my 5 sections (about 2-3 mm thick) prepared from this small placenta; probably there were somewhat more areolae. Hill et al. (1928) depicted 20 in their plate of an immature specimen (#8) and drew the interesting relationship to the uterine epithelium in cross section diagrams.
The placenta then is epitheliochorial in its maternal relationship and has a considerable number of discrete subchorionic spherical regions, the areolae that are depicted below. Similar placentas are often described in context with the placentas of pigs (e.g.: by Amoroso, 1961; Mossman, 1987; and Wooding & Flint, 1994). In their considerations, the trophoblast never fuses with endometrial epithelium and uterine ‘milk’ is produced by the endometrial glands and forwarded to “areolae” that are depicted by Amoroso and the other authors. These regions were described by Amoroso on pp. 174-175 as follows:
“The most conspicuous structural differentiations on the surface of the pig’s chorion are the areolae. Embryologists have long been aware of the fact that these structures which arise as minute circular areas opposite the mouths of the uterine glands, represent regions of the chorion which may have become specialised in connection with the absorption of the secretions elaborated by the glands and the uterine epithelium (von Baer, 1828; Turner, 1875; Grosser, 1927). Together with the uterine depressions which fit over them, they form definite organic units isolated structurally from the rest of the placenta. Two morphological types of areolae (called “rosettes” by Abromavich, 1926) are differentiated on the chorion of the 10 to 12 mm embryo, the regular areolae, the ones usually described in the literature, which are circular, opaque, and complexly folded, cup-like invaginations of the chorionic surface (Fig. 15.25b), and the irregular areolae (called pits by Heuser, 1927), translucent plaque-like structures with little tendency to invagination, and as much as fifteen times the diameter of the regular areolae (Fig. 15.25a). During very early stages of gestation, the surface of the regular areolae is flat (Fig. 15.26a), but as gestation advances the flat surface is invaginated and becomes complicated by ridges and papilla-like structures (Fig. 15.26b). The two structural types, occurring in large numbers (8000-9000), are distributed over the entire chorion and are relatively more numerous in mid-pregnancy (approximately 6 per square centimetre) than at term (approximately 2.5 per square centimetre). Microscopically, the areolae have a different type of epithelium and vascular pattern from the interareolar area, details of which are described by Abromavich (1926); Goldstein, (1926); Heuser (1927); Brambel (1933); and Wislocki and Dempsey (1946b). The epithelial cells are mostly simple columnar. All have small darkly staining nuclei and a pale cytoplasm containing numerous vacuoles; the contents of which stain like the secretion (uterine milk) located in the areolar cups. The cells are further characterised by long lasting deposits of glycogen and dense concentrations of iron and acid phosphatase (Wislocki and Dempsey, 1946b). The blood supply to the folds in the wall of the cup comes from a capillary ring, linked with the capillaries in the interareolar area, and is drained by a number of venules depending on the number of making up the wall of the cup. In the areolae, the capillaries never displace the epithelial cells as they do in the chorionic ridges of the interareolar areas.”
In a photograph of this region from a ‘Loris’, Wooding and Flint (1994, p. 309) labeled the regions as ‘chorionic vesicles’ as did Amoroso when he considered a number of other species with similar structures. These authors showed them as being largely empty with short cristae emanating from the wall. Amoroso then went on to describe similar regions in a variety of varied animal placentas and dismissed their possible outcome as hippomanes in lemuroids, as had been speculated. The true function of areolae is only speculated upon but as yet unknown according to Wooding and Flint.
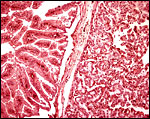
5) Details of fetal/maternal barrier
This is a typical epitheliochorial villous placenta with the trophoblast being single-layered and presumably directly apposed to the uterine epithelium. Placentas from similar types of animals show that there is no fusion of trophoblast and maternal endometrial epithelium.
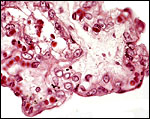 |
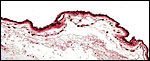
The surface of a villus is covered by a single layer of trophoblast, the capillaries have an exceedingly thin endothelium. |
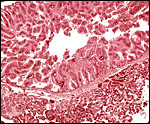
 |
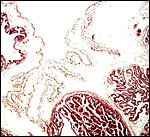
Surface of one villus with single layer of vacuolated epithelium. |
6) Umbilical cord
No information is available from the literature on cord length or structure of the pygmy loris placenta, but Hill et al. (1928) described it for N. lydekkerianus [=tardigradus]. The umbilical cord of this placenta was 3 cm long and had a minimal left spiral. The surface was smooth and without caruncles. There were two arteries and one vein, in addition to an allantoic duct.
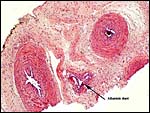 |
Umbilical cord with three vessels and allantoic duct. |
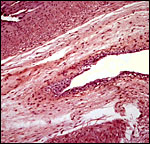 |
Allantoic duct in horizontal orientation. |
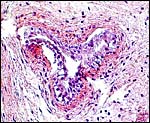 |
Section of the allantoic duct lined by urothelium. |
7) Uteroplacental circulation
No attached placenta was available and thus the general circulation, other than the fetal circulation, can be described. The latter has been the topic of detailed description by Hill et al. (1928).
8) Extraplacental membranes
A large, lobed allantoic sac is present only some portions of which are vascularized. The amnion is avascular and has a thin squamous epithelium on delicate connective tissue.
9) Trophoblast external to barrier
There is no extravillous trophoblast in this epithelio-chorial placenta.
10) Endometrium
The uterus is bicornuate and no decidua forms in pregnancy (Mossman, 1987).
11) Various features
None exist so far as is known other than the descriptions of 3 embryos of N. lydekkerianus by Hill et al. (1928) which focuses much on the development of the vitelline and allantoic circulations as well as the membrane development.
12) Endocrinology
There are reports (cited by Mossman & Duke, 1973) that adult lorisidae have a fetal type of ovarian structure with continued oogenesis. Estrone was determined in fecal specimens and it was found to be useful in determining the number of offspring and the beginning of pregnancy termination; the authors also evaluated aromatase activity in the placenta (Jurke et al., 1997; 1998). The length of pregnancy was thus suggested to be 187-198 days.
13) Genetics
The pygmy slow loris has 50 chromosomes with distinct sex chromosomes. The first delineation of the cytogenetics came from Chu & Bender (1962). A Giemsa-banded karyotype from the collection at CRES (San Diego Zoo) was published by O’Brien et al. (2006). Although N. coucang also possesses 50 chromosomes of a similar structure, hybrids have not been described. Molecular data (mtDNA) have been made available by Roos (2004) which justify the designation of three Nycticebus species. The evolution of growth hormone (GH) and prolactin was studied by Wallis et al. (2001; 2005); the results suggested rapid changes and lack of GH gene duplication in loris. Similarly, prolactin had an ‘episodic pattern’ of evolution. Other genetic studies may be found in papers by Su et al. (1997) and Hacia et al. (1998).
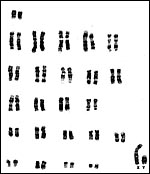 |
Chromosomes (2n=50) from CRES at San Diego Zoo. |
14) Immunology
MHC class I cDNA sequences were delineated for a number of primates by Flügge et al. (2002).
15) Pathological features
The stillbirth of a twin has been described by Jurke et al. (1998).
16) Physiologic data
Groves (2004) makes the point that ‘no taxonomic statements should be made on the basis of pelage characters’ because of the considerable variability (see Streicher, 2003; 2004). Ravosa (1998) provided results from cranial and dental measurements of the two species of loris and found the results to supports Groves’ original classification. An interesting study comes from Fisher et al. (2003) that suggests that a familiarity with the odor of males enhances breeding success. Fitch-Snyder & Ehrlich (2003) compared the mother-infant interactions between two different loris species and found them to be similar.
17) Other resources
Fibroblast cell lines are available from CRES by contacting Dr. Oliver Ryder at: oryder@ucsd.edu.
18) Other remarks – What additional Information is needed?
Early implantational descriptions are badly needed, as is a mature placenta that is still attached to the uterus.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society of San Diego. I am most grateful to Dr. Tilo Nadler of the Endangered Primate Rescue Center (EPRC) of Cuc Phuong, Vietnam for saving this placenta and making it available.
References
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall’s Physiology of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown & Co. Boston, 1961.
Chu, E.H.Y. and Bender, M.A.: Cytogenetics and evolution of primates. Ann. N.Y. Acad. Sci.102:253, 1962.
Enders, A.C. and Carter, A.M.: Comparative placentation: Some interesting modifications for histotrophic nutrition – a review. [This is a complicated reference]. Trophoblast Research 20:S1-S144, April 2006 – or: Placenta 27 Suppl. A, April 2006.
Fisher, H.S., Swaisgood, R.R. and Fitch-Snyder, H.: Odor familiarity and female preferences for males in a threatened primate, the pygmy loris Nycticebus pygmaeus: applications for genetic management of small populations. Naturwissenschaften 90:509-512, 2003.
Fitch-Snyder, H. and Ehrlich, A.: Mother-infant interactions in slow lorises (Nycticebus bengalensis) and pygmy lorises (Nycticebus pygmaeus). Folia Primatol. (Basel) 74:259-271, 2003.
Flügge, P., Zimmermann, E., Hughes, A.L. Günther, E. and Walter, L.: Characterization and phylogenetic relationship of prosimian MHC class I genes. J. Mol. Evol. 55:768-775, 2002.
Groves, C.: Primate Taxonomy. Smithsonian Series in Comparative Evolutionary Biology. Washington, DC 2001.
Groves, C.P.: Taxonomy and biogeography of primates in Vietnam and neighboring regions. Chapter 3 (pp.15-22) in Nadler, T., Streicher, U. and Long, H.T.: Conservation of Primates in Vietnam. Haki Publishing, Hanoi, 2004.
Hacia, J.G., Makalowski, W., Edgemon, K., Erdos, M.R., Robbins, C.M., Fodor, S.P., Brody, L.C. and Collins, F.S.: Evolutionary sequence comparisons using high-density oligonucleotide arrays. Nature Genet. 18:155-158, 1998.
Hill, J.P., Ince, F.E. and Rau, A.S.: The development of the foetal membranes in Loris, with special reference to the mode of vascularisation of the chorion in the Lemuroides and its phylogenetic significance. Proc. Zool. Soc. London pp. 699-718, 1928.
Jurke, M.H., Czekala, N.M. and Fitch-Snyder, H.: Non-invasive detection and monitoring of estrus, pregnancy and the postpartum period in pygmy loris (Nycticebus pygmaeus). Amer. J. Primatol. 41:103-115, 1997.
Jurke, M.H., Czekala, N.M., Jurke, S., Hagey, L.R., Lance, V.A., Conley, A.J. and Fitch-Snyder, H.: Monitoring pregnancy in twinning pygmy loris (Nycticebus pygmaeus). Amer. J. Primatol. 46:173-183, 1998.
Kappeler, P.M.: Patterns of sexual dimorphism in body weight among prosimian primates. Folia Primatol. (Basel) 57:132-146, 1991.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nowak, R.M.: Walker’s Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
O’Brien, S.J., Menninger, J.C. and Nash, W.G., eds.: Atlas of Mammalian Chromosomes. Wiley-Liss; A. John Wiley & Sons, Inc. Hoboken, N.J., 2006.
Ravosa, M.J.: Cranial allometry and geographic variation in slow lorises (Nycticebus). Amer. J. Primatol. 45:225-243, 1998, and 47:89, 1999.
Roos, C.: Molecular evolution and systematics of Vietnamese primates. Chapter 4 (pp.23-28) in Nadler, T., Streicher, U. and Long, H.T.: Conservation of Primates in Vietnam. Haki Publishing, Hanoi, 2004.
Streicher, U.: Saisonale Veränderungen in Fellfärbung und Fellzeichnung beim Zwergplumplori (Nycticebus pygmaeus) und ihre taxonomische Bedeutung. Zool. Garten 73:368-373, 2003.
Streicher, U.: Seasonal changes in the colouration and fur patterns in the pygmy loris (Nycticebus pygmaeus). Chapter 5 (pp.29-32) in Nadler, T., Streicher, U. and Long, H.T.: Conservation of Primates in Vietnam. Haki Publishing, Hanoi, 2004.
Su, B., Wang, W., Lan, H. and Zhang, Y.: Genetic diversity and phylogenetic relationships among Chinese Macacas based on protein electrophoresis. Yi Chuan Xue Bao 24:109-115, 1997.
Wallis, O.C., Mac-Kwashie, A.O., Makri, G. and Wallis, M.: Molecular evolution of prolactin in primates. J. Mol. Evol. 60:606-614, 2005.
Wallis, O.C., Zhang, Y.P. and Wallis, M.: Molecular evolution of GH in primates: characterization of the GH genes from slow loris and marmoset defines an episode of rapid evolutionary change. J. Mol. Endocrinol. 26:236-258, 2001.
Weigl, R.: Longevity of Mammals in Captivity; From the Living Collections of the World. [Kleine Senckenberg-Reihe 48]. E. Schweizerbart’sche Verlagsbuchhandlung (Nägele und Obermiller), Stuttgart, 2005.
Wooding, F.B.P. and Flint, A.P.F.: Placentation. Chapter 4 (pp.233-460) in, G.E. Lamming, ed. Marshall’s Physiology of Reproduction, 4th ed. Vol. 3, Part 1. Chapman & Hall, London, 1994.