| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last
updated: November 28, 2003 |
Antilocapra americana
Order: Artiodactyla
Family: Antilocapridae
1) General Zoological Data
This single member of the genus is widely distributed over the American west and northern Mexico. In the distant past, thirteen species of antilocapridae were found in North America, this is the only remaining species of this genus, with five recognized subspecies. They are well known for their speed and also difficulty in keeping healthy in zoological parks. Longevity in captivity is 12+ years. They are said to be the only animal with both horn and antler, having a hollow horn over a protrusion of bone. Their name derives from the short prong at the distal antler. Beintema et al. (2003) sought gene for ribonuclease gene sequences and were unable to detect the seminal-type ribonuclease in pronghorn, apparently having completely deleted in pronghorn, presenting difficulties for phylogenetic assignment. Indeed, Nowak (1999) summarized recent information on relationship to other taxa that suggests they be comprised with cervids under the superfamily Cervoidea. They weigh up to 70 kg, males being larger than females. Both have antlers. Neonates weigh from 3-5.78 kg (Hayssen et al.1993). A comprehensive review of all aspects of this species has been published by one the best experts on pronghorn, O'Gara (1978).
Aerial surveys of the population in Wyoming, for instance, were conducted by Johnson et al. (1991) with several herd sizes over 300 animals being detected. In Mexico, however, the three subspecies of pronghorn are considered to be significantly endangered with only a few thousand animals remaining (Ceballos & Navarro, 1991). This is especially true for the subspecies of Baja California.
 |
Pronghorn antelope. |
Wislocki & Fawcett described four mid-pregnant uteri, but a more comprehensive study of female reproduction was undertaken by O'Gara (1969). He indicated that between three to seven ova developed into expanded blastocysts, twins resulted as a general rule. The expanding tip of the proximal, normally implanted gestational sac pierced by the necrotic tip of its membranes the distal embryonic sac, thus eliminating excess implantation. Gestation is around 250 days with singletons in the first pregnancy and twins born thereafter in more than 50% of pregnancies. Triplets have also been reported. Transuterine migration occurs. Neonates suffer high mortality from predation. Possible monozygotic twins occur (see section on pathology).
3) Implantation
A few macroscopic descriptions of young implantations have been detailed by O'Gara (1969) but histology is needed. I have had available only one mid-pregnant uterus containing twins, made available by the Los Angeles zoo many years ago. The photographs come from that specimen.
4) General Characterization of the Placenta
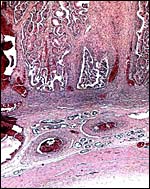
This is a typical polycotyledonary, epithelio-chorial placenta, similar to most other ungulates. The placenta has are an average of 92 (flat) cotyledons and the membranes meet in midline around 50-75 mm length (O'Gara, 1969). Anastomoses between fetal circulations are most uncommon. The distal tip of the membranes is necrotic. There is, however great variation in the number of placentomes developing, from 46 to 180 (O'Gara, 1969). Weights are not available of placentas and only volumes of cotyledons have been ascertained by O'Gara (1969).
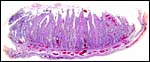 |
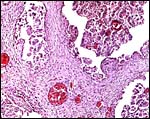
Implanted pronghorn cotyledon with uterus below and chorionic membrane above. |
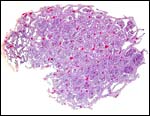 |
Cross section through cotyledon showing the complex network of maternal tissue and villi interdigitating. |
This is typically epitheliochorial with cuboidal trophoblast and a moderate number of binucleate trophoblast. Villi contain relatively little connective tissue. They are diffusely interposed between major uterine trabeculae.
The cord attaches nearer the cervix than the utero-tubal junction (Mossman, 1987), but there are no descriptions or measurements of umbilical cords in any of the descriptions listed here.
7) Uteroplacental circulation
No studies are found in the literature.
8) Extraplacental membranes
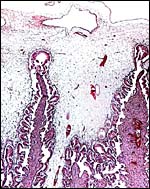
The peripheral tip towards to oviductal end of amnion/allantois usually becomes necrotic (yellow-cheesy) from lack of allantoic vasculature and "acts as a lethal weapon in intra-uterine competition" (O'Gara, 1969). Mossman (1987) described the allantois as a "long, narrow, tubular allantoic cavity".
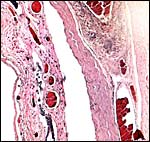 |
Chorion and (little) amnion at right, vascularized allantois at left. |
There is no uterine invasion by trophoblast.
10) Endometrium
The uterus is bicornuate; a cervical mucus plug develops during gestation. (O'Gara, 1969). Normally four rows of caruncles develop, although O'Gara (1969) found five uteri without caruncles in his large series. There is no true decidua. Between cotyledons, the endometrial glands continue to be active.
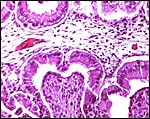
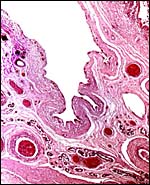 |
The edge of a cotyledon (right with endo/myometrium between the cotyledons. Numerous endometrial glands with sparse openings into uterine lumen. |
There is neither a subplacenta nor other unusual features of placentation.
12) Endocrinology
I have not been able to identify any studies on endocrinology of pronghorn.
13) Genetics
Pronghorns have 58 acrocentric chromosomes (Wurster & Benirschke, 1967; Hsu & Benirschke, 1969). Hybrids are unknown. Dain (1977) studied the replication pattern of the sex chromosomes and found it to be larger than the "normal" 5% of genome; they detected late-labeling portions of both X and Y that were in excess of the 4.7% for the "regular" X chromosome. Gallagher et al. (1994) compared the band homologies of several Bovidae, including the pronghorn and found cattle and pronghorn to be most similar. A study of satellite DNA was undertaken by Denome et al. (1994). Murray et al. (1995) were able to use mitochondrial D-loop variation for the species identification among many different ungulates. Lee et al. (1994) were able to differentiate some subspecies by allozyme analysis in a large population from different areas; their study of mtDNA variation however was less informative.
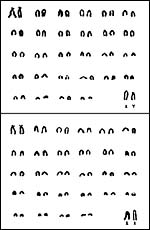 |
Karyotypes male and female pronghorns. |
The serological studies conducted are listed in the next section.
15) Pathological features
O'Gara (1969) depicted a "teratomas" which I believe to be more
likely an acardiac fetus (his fig. 17). In personal communications with
the author I learned that another observer had identified several same-sex
pronghorn twin fetuses with vascular anastomoses of their placental circulations,
suggesting that MZ twins may occur; thus, acardiacs would be a good possibility.
Griner (1983) autopsied 25 pronghorn and the difficulty with immobilization
is shown in his data. "Lumpy jaw" and various infections, as
well as "white muscle disease" were other causes of death. He
also saw one squamous cell carcinoma of the oral mucosa with metastases.
Experimental high selenium diets had no significant impact on captive
pronghorn (Raisbeck et al., 1996). An apparent copper deficiency in hand-reared
fawns disappeared after copper supplementation (Miller et al., 2001).
Polioencephalomalacia in some Saskatchewan wild pronghorn (after grain
ingestion) was attributed to thiamine deficiency by Wobeser et al. (1983).
Numerous studies of infectious agents have been undertaken in captive
and wild pronghorn. They can be separated into three categories:
1) Parasites: Dubey (1980; 1981) identified Sarcocystis
and Toxoplasma in pronghorn. Kuttler (1984) found Anaplasma
marginale. Kingston et al. (1990) identified Entamoeba bovis
in the feces of pronghorn fawns. Simmons et al. (2002) identified natural
infection with Parastrongylus tenuis in four captive animals
of Nebraska that led to euthanasia.
2) Viruses: Stauber et al. (1980) surveyed for antibodies in
104 adult and 42 fawns and found antibodies to bovine infectious diarrhea,
infectious bovine rhinotracheitis, parainfluenza, bovine adenovirus 7
& 3 and anaplasma, but none to brucella, bluetongue or epizootic hemorrhagic
disease. Jessup (1985) studied antibodies against two orbiviruses (bluetongue)
and found it uncommonly. Bluetongue infection, however, caused epidemic
losses in Wyoming during epidemics of 1976 and 1984 (Thorne et al., 1988).
Infection by mosquito-borne Malpias spring virus was demonstrated by Clark
et al. (1988). A survey of antibodies to malignant catarrh fever virus
in several ruminants by Li et al. (1996) found none in pronghorn but it
was present in several other wild ungulates. A large outbreak of epizootic
hemorrhagic disease killed numerous pronghorn (Brodie et al., 1998). Experimental
infection with contagious ecthyma virus yielded oral lesions but had only
a "limited clinical response" and was not thought to impact
wild populations (Lance et al., 1983).
3) Bacteria: Griner (1983) already indicated losses from salmonellosis.
Deaths due to infection with Pasteurella multocida has been reported
from Oregon and Montana (Dunbar et al., 2000). Brucella abortus
found in Yellowstone bison rarely colonizes pregnant pronghorn according
to the survey by Elzer et al. (2002). An important description of fusobacteriosis
comes from Edwards et al. (2001). These recently captured animals were
housed in a pen previously used by cattle. Following a rain, pododermatitis
developed with rapid sepsis due to Fusobacterium necrophorum
ensuing.
16) Physiologic data
Hand-rearing and study of nutrition were studied in considerable detail
by Schwartz et al. (1976). Diarrhea and joint problems were major side
effects of some diets. Because of significant declines of pronghorn populations
in Oregon, Dunbar et al. (1999) studied numerous parameters of fawns and
adult animals, without finding a significant change to explain the decline.
Baker et al. (1998) studied diet supplements and their digestive efficiency
of several American ruminants, including the pronghorn. Hematologic values
and blood chemistry were studied by Clemens et al. (1987). Pronghorn were
found to have significantly higher selenium levels than bison and deer.
Problems with tranquilization of pronghorn were amply studied by Pusateri
et al. (1982) who found dosing with diazepam and promazine-HCl difficult.
In efforts to find a "model" for the study of sickle cell disease,
Dhindsa et al. (1975) studied sera of deer and pronghorn. They found low
alkaline phosphatase levels in pronghorn. Mossman & Duke (1973) stated
that the ovaries of pronghorn do not differ much from other ungulates
and depicted (their fig. 6.98) the two types of luteal cells they identified.
17)
Other resources
Cell strains of fibroblasts are available from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other remarks - What additional
Information is needed?
Timing of implantation and early microscopic studies are needed, as well
as endocrine studies.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Baker, D.L., Stout, G.W. and Miller, M.W.: A diet supplement for captive
wild ruminants. J. Zoo Wildl. Med. 29:150-156, 1998.
Beintema, J.J., Breukelman, H.J., Dubois, J.Y. and Warmels, H.W.: Phylogeny of ruminants secretory ribonuclease gene sequences of pronghorn (Antilocapra americana). Mol. Phylogenet. Evol. 26:18-25, 2003.
Brodie, S.J., Bardsley, K.D., Diem, K., Mecham, J.O., Norelius, S.E. and Wilson, W.C.: Epizootic hemorrhagic disease: analysis of tissues by amplification and in situ hybridization reveals widespread orbivirus infection at low copy numbers. J. Virol. 72:3863-3871, 1998.
Ceballos, G. and Navarro, L, D.: Diversity and conservation of Mexican mammals. Chapter 9 (pp. 167-198) in Biogeography and Biodiversity. M.A. Mares and D.J. Schmidly, eds. Univ. Oklahoma Press, Norman, Oklahoma, 1991.
Clark, G.G., Calisher, C.H., Crabbs, C.L., Canestorp, K.M., Tesh, R.B., Bowen, R.A. and Taylor, D.E.: Malpais spring virus: a new vesiculovirus from mosquitoes collected in New Mexico and evidence of infected indigenous and exotic ungulates. Amer. J. Trop. Med. Hyg. 39:586-592, 1988.
Clemens, E.T., Meyer, K.L., Carlson, M.P. and Schneider, N.R.: Hematology, blood chemistry and selenium values of captive pronghorn antelope, white-tailed deer and American bison. Comp. Biochem. Physiol. C 87:167-170, 1987.
Dain, A.: DNA replication in the sex chromosomes of the pronghorn and Rocky Mountain goat. Cytogenet. Cell Genet. 18:126-135, 1977.
Denome, R.M., O'Callaghan, B., Luitjens, C., Harper, E. and Bianco, R.: Characterization of a satellite DNA from Antilocapra americana. Gene 145:257-259, 1994.
Dhindsa, D.S., Cochran, T.H., Castro, A., Swanson, J.R. and Metcalfe, J.: Serum biochemical and electrophoretic values from four deer species and from pronghorn antelope. Amer. J. Vet. Res. 36:1455-1457, 1975.
Dubey, J.P.: Sarcocystis species in moose (Alces alces), bison (Bison bison), and pronghorn (Antilocapra americana) in Montana. Amer. J. Vet. Res. 41:2063-2065, 1980.
Dubey, J.P.: Isolation of encysted Toxoplasma gondii from musculature of moose and pronghorn in Montana. Amer. J. Vet. Res. 42:126-127, 1981.
Dunbar, M.R., Velarde, R., Gregg, M.A. and Bray, M.: Health evaluation of a pronghorn antelope population in Oregon. J. Wildl. Dis. 35:496-510, 1999.
Dunbar, M.R., Wolcott, M.J., Rimler, R.B. and Berlowski, B.M.: Septicemic pasteurellosis in free-ranging neonatal pronghorn in Oregon. J. Wildl. Dis. 36:383-388, 2000.
Edwards, J.F., Davis, D.S., Roffe, T.J., Ramiro-Ibanez, F. and Elzer, P.H.: Fusobacteriosis in captive wild-caught pronghorns (Antilocapra americana). Vet. Path. 38:549-552, 2001.
Elzer, P.H., Smith, J., Roffe, T., Kreeger, T., Edwards, J. and Davis, D.: Evaluation of Brucella abortus strain RB51 and strain 19 in pronghorn antelope. Ann. N.Y. Acad. Sci. 969:102-105, 2002.
Gallagher, D.S., Derr, J.N. and Womack, J.E.: Chromosome conservation among the advanced pecorans and determination of the primitive bovid karyotype. J. Hered. 85:204-210, 1994.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 3, Folio 136, 1969. Springer-Verlag, New York.
Jessup, D.A.: Epidemiology of two orbiviruses in California's native wild ruminants: preliminary report. Progr. Clin. Biol. Res. 178:53-65, 1985.
Johnson, B.K., Lindzey, F.G. and Guenzel, R.J.: Use of aerial line transect surveys to estimate pronghorn populations in Wyoming. Wildl. Soc. Bull. 19:315-321, 1991.
Kingston, N., Williams, E.S. and Thorne, E.T.: Invasive entamoebae in pronghorn (Antilocapra americana) from Wyoming. J. Wildl. Dis. 26:50-54, 1990.
Kuttler, K.L.: Anaplasma infections in wild and domestic ruminants: a review. J. Wildl. Dis. 20:12-20, 1984.
Lance, W.R., Hibler, C.P. and DeMartini, J.: Experimental contagious ecthyma in mule deer, white-tailed deer, pronghorn and wapiti. J. Wildl. Dis. 19:165-169, 1983.
Lee, T.E., Bickham, J.W. and Scott, M.D.: Mitochondrial DNA and allozyme analysis of North American pronghorn populations. J. Wildl. Manage. 58:307-318, 1994.
Li, H., Shen, D.T., Jessup, D.A., Knowles, D.P., Gorham, J.R., Thorne, T., O'Toole, D. and Crawford, T.B.: Prevalence of antibody to malignant catarrhal fever virus in wild and domestic ruminants by competitive-inhibition ELISA. J. Wildl. Dis. 32:437-443, 1996.
Miller, M., Amsel, S., Boehm, J. and Gonzales, B.: Presumptive copper deficiency in hand-reared captive pronghorn (Antilocapra americana). J. Zoo Wildl. Med. 32:373-378, 2001.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Murray, B.W., McClymont, R.A. and Strobeck, C.: Forensic identification of ungulate species using restriction digests of PCR-amplified mitochondrial DNA. J. Forensic Sci. 40:943-951, 1995.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
O'Gara, B.W.: Unique aspects of reproduction in the female pronghorn (Antilocapra americana Ord). Amer. J. Anat. 125:217-232, 1969.
O'Gara, B.W.: Antilocapra americana. Mamm. Species 90:1-7, 1978.
Pusateri, F.M., Hibler, C.P. and Pojar, T.M.: Oral administration of diazepam and promazine hydrochloride to immobilize pronghorn. J. Wildl. Dis. 18:9-16, 1982.
Raisbeck, M.F., O'Toole, D., Schamber, R.A., Belden, E.L. and Robinson, L.J.: Toxicologic evaluation of a high-selenium hay diet in captive pronghorn antelope (Antilocapra americana). J. Wildl. Dis. 32:9-16, 1996.
Schwartz, C.C., Nagy, J.G. and Kerr, S.M.: Rearing and training pronghorns for ecological studies. J. Wildl. Managem. 40:464-468, 1976.
Simmons, H.A., Steffen, D.J., Armstrong, D.L. and Rogers, D.G.: Parastrongylus tenuis in captive pronghorn antelope (Antilocapra americana) in Nebraska. J. Wildl., Dis. 38:822-825, 2002.
Stauber, E.H., Autenrieth, R., Markham, O.D. and Whitbeck, V.: A seroepidemiologic survey of three pronghorn (Antilocapra americana) populations in southeastern Idaho, 1975-1977. J. Wildl. Dis. 16:109-115, 1980.
Thorne, E.T., Williams, E.S., Spraker, T.R., Helms, W. and Segerstrom, T.: Bluetongue in free-ranging antelope (Antilocapra americana) in Wyoming: 1976 and 1984. J. Wildl. Dis. 24:113-119, 1988.
Wislocki, G.B. and Fawcett, D.W.: The placentation of the pronghorned antelope (Antilocapra americana). Bull. Museum Comp. Zool. Harvard College 101:548-558, 1949
Wobeser, G., Daoust, P.Y. and Hunt, H.M.: Polioencephalomalacia-like disease in pronghorns (Antilocapra americana). J. Wildl. Dis. 19:248-252, 1983.
Wurster, D.H. and
Benirschke, K.: Chromosome studies on some deer, the springbok, and the
pronghorn with notes on placentation in deer. Cytologia 32:273-285, 1967.