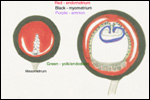
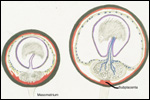
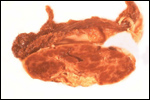
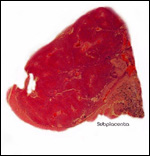
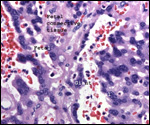
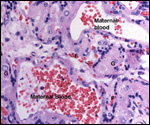
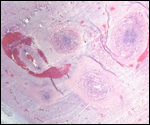
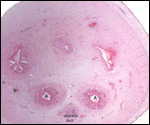
|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
Erethizon dorsatum
Order: Rodentia
Family: Erethizontidae
1) General zoological data of species
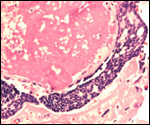
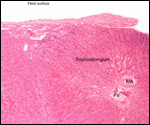
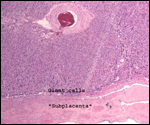
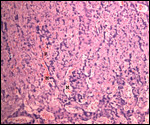
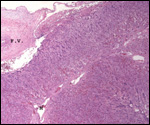
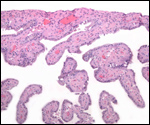
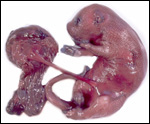
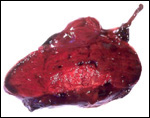
This single species (with 6 possible subspecies) is indigenous to North America and represents its only porcupine species. Its habitat is largely woods, with a strong arboreal tendency. It is strictly herbivorous with an appetite for bark and salty woods. The animal is mostly nocturnal and is not closely related to the African porcupines, both having arisen by converging evolution from other Rodentia (Wood, 1950). On the other hand, when Luckett & Mossman (1981) studied the placentas of Hystrix and another hystricognath rodent, Bathyergus, they found them to have a great similarity in their placentation with the South American hystricomorph species. This, among other aspects, has given rise to a great controversy over the possible monophyly or development by converging evolution of porcupines.
I believe, however, that the originally South American origin of this hystricomorph rodent is strongly supported by its having an X-chromosome that is, as is true for other South American rodents, of twice the size of all other rodents. While captive breeding is often successful and newborns become easily tractable, we are not aware of any colonies other than the animals that are kept in zoological gardens. Because of its preference for trees, Europeans refer to these animals as "Urson" (in German "Baumstachler"). The modified hair consists of quills with fine hooks at the end. Further references to its anatomy are listed by Hayssen et al. (1993). The longevity of American porcupines is at least 10 years.