| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: June 7, 2004. |
Chaetodipus (Perognathus) fallax
Order: Rodentia
Family: Heteromyidae
1) General Zoological Data
Heteromyidae comprise pocket mice and kangaroo rats, and the species discussed here is one of the pocket mice of the Western USA . While we are sure that this species belongs to the subgenus Chaetodipus that comprises 15 Perognathus species, its exact species designation is only tentative and is currently under taxonomic scrutiny. Pocket mice have fur-lined external cheek pouches, whence they derive their name (Gr. pera = pouch; gnathos = mouth; Gotch, 1979) and live in burrows, subsisting on seeds etc. They are nocturnally active (Nowak, 1999). Hall & Kelson (1959) have provided an extensive description of this very heterogeneous group of rodents that possess numerous designated subspecies and also has many karyotypes.
This animal was trapped in the San Pasqual Wild Animal Park, San Diego County , California ; it weighed 25 g. It was made available to me by Dr. V. Lance of CRES at the San Diego Zoo. Both uterine horns were pregnant, but several fetuses were found to have been resorbed when the uterus was opened. Moreover, because of the heat exposure after trapping, there was moderate autolysis. Thus, the preparations are suboptimal. Future trapping is underway.
 |
Gross appearance of this pocket mouse after its back had been shaven for skin biopsy. |
2) General Gestational Data
Bronson (1998) described the reproductive physiology thus: “…all heteromyids are polyestrus, producing litters of two to eight young. The length of the estrous cycle varies from 4 or 5 days in some species to several weeks in others. At least some species have a postpartum estrus. Gestation for most heteromyids is on the order of 3 or 4 weeks”. Hayssen et al. (1993) listed newborn size as around 1.5 g for several species of pocket mice and indicated also that several pocket mouse species have litters 1-2 times a year. Placental weights are unknown.
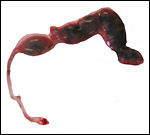 |
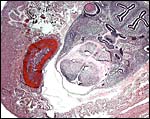
Uterus with six immature gestations; only one is in the left horn that had at least three resorption sites. |
3) Implantation
I am not aware of an accurate descriptions of early stages of implantation in this species but assume that they must be similar to those of the domestic mouse (see that chapter). Perhaps the easiest overview to read about the complex development of mouse placentation is the review by Kaufman (1992) because of the profuse usage of sequential photographs and diagrams in this book. A detailed but general description of adhesion, infiltration and early placentation of rodents is available from Abrahamsohn & Zorn (1993) who concentrated on decidualization in domestic mice. Nevertheless, it is likely that pocket mice are generally similar, but they have not been studied in greater detail. Thus, in the Mouse chapter I wrote this on domestic mouse placentation: “ It is a superficially invasive placenta (it is "interstitial"), with a "folding" type of amniogenesis. The major portion of the placenta becomes discoid and has both - a larger labyrinthine (hemochorial) structure - and a smaller region, the trophospongium. Both of these areas are especially confusing. In addition to these two areas, there is the portion of the gestational sac represented by the inverted yolk sac. It forms the dome of the gestational sac. All these structures are substantially different. In early stages, the yolk sac is well developed. Later, however, it becomes inverted, so that its endodermal epithelial layer comes to lie opposite the endometrium on the uterine wall. The conceptus' membranous sac, lying opposite to the disk, thus becomes the "visceral yolk sac placenta".
In the early stages of the developing mouse blastocyst, the trophectoderm represents the major portion (70-80% of cells) of the future conceptus. A smaller number of inner cells (the currently much debated “stem cells”) that represent the future fetus lie within the blastocyst. The initial attachment of the blastocyst, which is derived from the trophectoderm – the “mural” trophectoderm, occurs when trophoblast invades the endometrium. The implanting blastocyst is then still positioned deep in an endometrial crypt. This proliferating trophoblast forms a "cone" herein that is often referred to as the "Träger" (= carrier). When the opening of the endometrial crypt closes , this area becomes the egg chamber ("Eikammer").
The blastocyst, with its proliferating trophoblast, now assumes a more cylindrical shape. The initial attaching trophoblast cells proliferate from the bottom of the cone as a modification or extension of the träger. During this early stage, the embryonic yolk sac makes up another dominant portion of the developing embryonic tissues. Later, this yolk sac membrane becomes totally inverted, so that its epithelium presses against the endometrial mucosa at the dome of the cavity. It has recently been shown that fibrinogen is essential for the successful attachment the blastocyst and maintenance of placentation (Iwaki et al., 2002). Mice deficient in the FG-? chain consistently aborted.
The placental disk now lies on the opposite (mesometrial) side of the uterus. Although there is no allantoic sac in mice, some of the initially present allantoic tissues provide the stalk for the development of the umbilical cord. The early fusion of the allantoic and chorionic murine membranes has now been studied in some detail by Downs (2002). The fusion process appears to be highly regulated through the attractions of the adhesion molecule VCAM-1 in the allantois and a4-integrin in the chorion. After fusion the remaining barrier is broken down, and thus, the body stalk develops. In comparing the development of different mouse strains, Dickson (1967) found that blastocysts do not always develop equally well and also, that their development is not necessarily synchronous. Recent research has identified a variety of genes that are responsible for implantation, adhesion, endocrine response, and so forth. They are summarized by Giudice (1999).
As was indicated at the beginning of this chapter, the anatomy of the mouse placenta is very complex. Rossant & Croy (1985) used reconstituted embryos to determine which cells of the placenta are of maternal origin, which are fetal and, of these, which originate from the embryonic disk and which come from the trophectoderm. Cross et al. (1994) provided some initial comparisons of mouse and human implantation and detailed the molecular basis for the interactions of the maternal and fetal organisms.
A major publication by Georgiades et al. (2002) presents much more detailed information on the cellular composition of the placental disk and on the implantation site. It endeavors especially to compare the various cell types of mouse trophoblast with those found in the human placenta. The original publication must be consulted for this detail. Likewise, the review by Rossant & Cross (2001) provides evidence that extensive signaling occurs between trophoblast and embryonic cells which, when disrupted, may lead to abortion or embryonic maldevelopment. Cross (2000a) has given further emphasis to the notion that the mouse placentation is a useful model to understand the less-accessible (genetically speaking) human placentation.
The ectoplacental cone and the extraembryonic ectoderm of the primitive streak stage are derived from the trophectoderm. These experiments showed that, when the placental portions were divided into labyrinthine and spongiotrophoblastic components, the maternal contributions were essentially confined to the latter segments. Most of the decidual tissue was maternal in origin but there was also a minor fetal tissue contamination ( i.e . the trophoblastic giant cells). About 30% of the cells in the placental disk derive from maternal tissues. They are largely confined to the spongiotrophoblast region. The cellular components of the inner cell mass, which is largely confined to the placental labyrinth, constitute only about 4%. The analysis was somewhat hampered by the presence of contaminating blood cells but are the best approximations available. These are difficult experiments. It should also be mentioned that not all placentas of reconstituted embryos were identically admixed. Hence, not surprisingly, other investigators had somewhat different findings
The giant cells of the rodent placenta have presented a special topic of study for many investigators. These cells, which form the primary site of attachment to the endometrium, can be grown in vitro (Copp, 1980). Their development begins at the abembryonic pole of trophoblast and then spreads towards the embryo (+/- 4dpc). During the first half of development, 10-30% of trophoblastic cells contain giant nuclei that have large quantities of DNA. This DNA is derived through the process of endomitosis (chromosomal replication without cell division). By 11 dpc, up to 850 times the normal amount of DNA is found in these nuclei (Barlow & Sherman, 1972; 1974). DNA replication continues unabated throughout gestation (Copp, 1978). Experiments with actinomycin D by Snow & Ansell (1974) indicate that the DNA in these giant cells is actually composed of large numbers of chromosomes. Mitosis does not occur in these cells (Heine, 1976). Dickson (1963) found that, in delayed implantation, fewer of these cells develop.
Not only do these cells accomplish the initial implantation; they also acquire endocrine function (Gardner & Johnson, 1972), immune functions and cytokine production (Cross, 2000 b ). Ultimately, the giant cells surround the entire membranes. They become the trophospongium , the area of the placental disk located at the "bottom", at the site of implantation and connecting the placenta to the decidua. This tissue is largely composed of giant cells. Its cells are more compacted at the interface between the peripheral giant cell layer and the labyrinth (please see subsequent photo). Through mutational analysis, gene insertion, knockouts, and other modern techniques, we have obtained a much better understanding of the intricate nature of the implantation process and early development (see section on genetics below. Detailed accounts are by Anson-Cartwright et al., 2000; Cross 2000; Luo et al., 1997).
The placental labyrinth comprises the major portion of the placental disk; it has very thin fetal capillaries that are supported by a miniscule amount of connective tissue. They, in turn, are surrounded by trophoblast. And these are bathed in the maternal sinusoidal blood. This region constitutes the major site of maternal/fetal exchange. The labyrinth forms by much folding of the villous tissues.
The visceral yolk sac placenta , the thin membrane that lies opposite the placental disk and apposes the peripheral endometrium, is much more simply constructed. A cylindrical epithelium faces the variably cuboidal and cylindrical endometrial epithelium, seemingly without having direct contact; at least this is difficult to ascertain in the sections made after fixation. Those apparent spaces seen in our routine histologic sections are artifact. They are caused by fixation-induced tissue shrinkage. The tissue components are actually contiguous. Important transport of many substances occurs at this interface. They are different, however, than those that reach the embryo through the disk. Brambell (1966) showed that immunoglobulins traverse this barrier from mother to fetus. And since then, many other substances have been shown to pass this epithelial contact zone.”
4) General Characterization of the Placenta
Heteromyidae, as other rodents, have an inverted yolk sac placentation in which the single disk is mesometrially oriented. There is a narrow stalk that connects the disk to the subplacenta in which numerous very large giant cells are found. Mossman (1973) wrote of Perognathus the following: “…very large giant cells do occur in both the abplacental and adplacental regions. This genus also has unusual, villus-like ridges over these areas of the endometrium, and these interdigitate with equally complex villi and folds of the inverted yolk sac (his Fig. 31.8). It is interesting that in the regions of this intimate association between endometrium and yolk sac the uterine epithelium is mainly low cuboidal and the yolk sac endoderm adjacent to it is so low that it appears squamous by light microscopy rather than high columnar as in most other rodents. This suggests a direct and rapid transfer between the maternal and fetal vascular systems of this area. Comparison by electron microscopy of this inverted yolk sac placentation of Perognathus with that of other rodents would be worthwhile.”
While nothing is known about the genetics of implantation in Perognathus , those of the domestic mouse are now gradually becoming known (see Hemberger & Cross, 2001).
5) Details of fetal/maternal barrier
Allen Enders (in litt., 2004) was kind enough to point out that all heteromyid rodents so far studied have had an endothelio-chorial placentation but also, that early implantation has not been described. In any event, the placentation differs from that of myomorph rodents according to his own observations.
Ramsey (1975) described the complex trophoblast development of the mouse thus:
" The trophoblast forming the ectoplacental cone or träger gives rise to three types of trophoblast. The earliest to appear are wandering giant cells that infiltrate the maternal tissue and eventually come in contact with maternal blood vessels which they open up, thereby creating the hemochorial state. These cells appear to be actively phagocytic for the first 10 days of gestation. The second derivative of the träger is syncytiotrophoblast and the final one the cytotrophoblast. These two compose the walls of the labyrinthine tubules."
(She then refers to an electronmicrophotograph showing this triple layer of trophoblast). Thus, the barrier in the disk is, strictly speaking, a hemotrichorial one. This, of course, is in contrast to the visceral yolk sac placental membrane, which is epithelium to epithelium and, despite this seemingly thick barrier, still provides much exchange function.
6) Umbilical cord
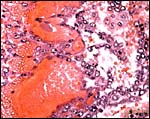
In these very young embryos, the umbilical cords were difficult to define, and their lengths could not be measured. The vessels contained abundant nucleated red blood cells. Two vessels each for allantoic and vitelline circulation were identified.
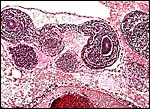 |
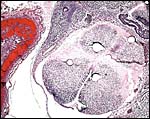
Cross section through abdomen of one of the gestations with umbilical cords of allantoic and vitelline circulations. |
7) Uteroplacental circulation
There are no publications of which I am aware. Undoubtedly it is similar to that of the domestic mouse (see chapter on Mouse) where I said this: “ The principal maternal vessels enter in the center of the ovoid disk. The arteriolar walls of the maternal vessels are modified by ingrowth of trophoblast. The veins are large and thin-walled, without trophoblast. Large maternal blood channels are situated deep in the labyrinthine tissue whence they divide to make the complex sinusoidal path along the villous surface. They then contact trophoblast.
Cross et al. (2001) have studied the vascularization of the mouse placenta with vascular casts. They suggested that the uterine NK cells are vital in establishing the vascular bed and that much of the vascular development depends on the activity of the giant cells. They found that the uterine artery branches into several spiral arteries. These vessels lack smooth muscle and converge at the junction to the giant cells. Here they open into the trophoblast-lined canals that enter the labyrinth. Mossman (1987) has diagrammed this complex blood flow.”
8) Extraplacental membranes
No decidua capsularis forms and there is no allantoic sac. The important structure is the inverted yolk sac, as detailed earlier. Unfortunately, the preservation of this placenta is insufficient for detailed observations and photographs.
9) Trophoblast external to barrier
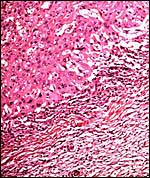
Giant cells are abundant, principally in the subplacenta but they are also present at the inverted yolk sac pole, as was depicted by Mossman (1973). No trophoblast was found in the myometrium or its vessels but it is intermixed with the decidua at the site of implantation.
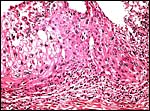 |
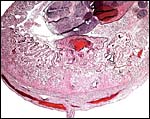
Another view of the large giant cells at the base, next to the myometrium (below). They are polyploid and often multinucleated. |
10) Endometrium
Mice make good decidua during gestation as well as artificially, when hormonally and physically stimulated. Presumably pocket mice fall into the same category but this has not been studied in detail. The large review of Abrahamsohn & Zorn (1993) considers these aspects of rodents in general and more specifically the authors pay attention to the better-studied mice. The decidua in the present case is present but it was too autolyzed for me to identify the various cellular elements mentioned by these authors as populating the decidua in rodents.
11) Various features
There is a subplacenta connected with a thin stalk to the main disk and it is characterized by abundant, large giant trophoblastic cells that possess a pale cytoplasm. This is more pronounced than is the case of the domestic mouse.
12) Endocrinology
The ovaries of pocket mice have apparently not been examined frequently; Mossman & Duke (1973) make reference to their similarity to Geomyidae in that they have an unusually thick theca interna of the follicles. No endocrine studies are listed in various publications that I have perused.
13) Genetics
The first author to study the California pocket mouse chromosomes was Cross (1931). He found 44 chromosomes in testis preparations of P. fallax fallax . Somatic chromosomes of pocket mice, however, were first studied in some detail by Patton (1967, 1972, 1977). The karyotypes of his first study were kindly made available for the Atlas by Hsu & Benirschke (1967-1975) as: P. arenarius (2n=42), P. intermedius (2n=46), P. penicillatus (2n=46), P. spinatus (2n=44). Patton (1967) also studied P. pernix (2n=38), P. goldmani (2n=52), P. artus (2n=54), P. baileyi (2n=48) and P. hispidus (2n=34). Patton also confirmed Cross' findings from two of his own specimens and he discussed Robertsonian fusion as a primary evolutionary driving force. Later, Patton (1977) examined the B-chromosome system of a related species, Perognathus baileyi . B-chromosomes (“supernumerary”) were heterochromatic, composed of two types, and only type I (the large ones) had chiasmata. There were also prominent blocks of C-banded heterochromatin at centromeres, but not the Y chromosome. Wahrman et al. (1969), who suggested that six chromosomal types had been identified in pocket mice, made the point that no hybrids had been described in pocket mice, perhaps because of their limited vagility. Nadler (1969) in the same volume, however, reported on hybridization of P. penicillatus (2n=46) and P. pernix (2n=52) with two animals having 2n=49. Mutations of the melanocortin-1-receptor gene may be responsible for the adaptive melanism that occurs in these species. Nachman et al. (2003) examined the genes regulating coat color of pocket mice.
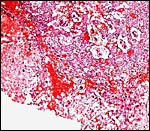
This pocket mouse has 44 chromosomes. It is the first time that this species has been studied karyologically with modern staining procedures. Unfortunately, the embryos did not grow, thus neither X-chromosome nor Y chromosome are defined. In other pocket mice the X is described as a large submetacentric element.
 |
Karyotype of female pocket mouse used in this study. |
 |
Female pocket mouse karyotype of dam used in this study. The sex chromosomes are no defined. “C-banding”. |
14) Immunology
No immunological studies in pocket mice are known to me.
15) Pathological features
Parasitism with coccidian ( Eimeria ) has been described in a variety of related rodents, including C. penicillatus (McAllister et al., 1991). Pneumocystis carinii infection was described in C. californicus by Laakkonen et al. (2001).
16) Physiologic data
I am not aware that any studies have been done.
17) Other resources
Cell strains of this maternal animal are available from Dr. O Ryder (oryder@ucsd.edu). Although the embryos showed initial cellular growth, they did not succeed ultimately.
18) Other remarks – What additional Information is needed?
Early stages of implantation, good endocrinology, and other physiological parameters need study.
Acknowledgement
I am grateful to Dr. V. Lance for submitting this specimen and to the genetics staff at CRES for preparing the karyotypes.
References
Abrahamsohn, P.A. and Zorn, T.M.T.: Implantation and decidualization in rodents. J. Exp. Zool. 266:603-628, 1993.
Anson-Cartwright, L., Dawson , K., Holmyard, D., Fisher, S.J., Lazzarini, R.A. and Cross, J.C.: The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nature Genetics 25:311-314, 2000.
Brambell, F.W.R.: The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet 2:1087-1093, 1966.
Bronson, F.H.: Rodentia. Pp. 282-289, in Encyclopedia of Reproduction, Vol. IV. E. Knobil and J.D. Neill, eds. Academic Press, San Diego , 1998.
Copp, A.J.: Interaction between inner cell mass and trophectoderm of the mouse blastocyst. I. A study of cellular proliferation. J. Embryol. Exp. Morphol. 48:109-125, 1978.
Copp, A.J.: The development of field vole ( Microtus agrestis ) and mouse blastocysts in vitro: A study of trophoblast cell migration. Placenta 1:47-60, 1980.
Cross, J.C.: A comparative study of the chromosomes of rodents. J. Morphol. & Physiol. 52:373-401, 1931.
Cross, J.C.: The mouse as a model for investigation of placental function. Chapter 5 (pp.51-60) in, The Placenta: Basic Science and Clinical Practice. J. Kingdom, E. Jauniaux and S. O'Brien, eds. Royal College of Obstetricians and Gynaecologists (RCOG) Press, London, 2000a.
Cross, J.C.: Genetic insights into trophoblast differentiation and placental morphogenesis. Seminars Cell Developm. Biol. 11:105-112, 2000b.
Dickson, A.D.: Trophoblastic giant cell transformation of mouse blastocysts. J. Reprod. Fertil. 6:465-466, 1963.
Dickson, A.D.: Variations in development of mouse blastocysts. J. Anat. 101:263-267, 1967.
Downs , K.M.: Early placental ontogeny in the mouse. Placenta 23:116-131, 2002.
Gardner , R.L. and Johnson, M.H.: An investigation of inner cell mass and trophoblast tissues following their isolation from the mouse blastocyst. J. Embryol. Exp. Morphol. 28:279-312, 1972.
Georgiades, P., Ferguson-Smith, A.C. and Burton , G.J.: Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23:3-19, 2002.
Giudice, L.C.: Genes associated with embryonic attachment and implantation and the role of progesterone. J. Reprod. Med. 44:165-171, 1999 (Suppl. 2).
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole , Dorset , UK , 1979.
Hall, E.R. and Kelson, K.R.: The Mammals of North America . The Rondal Press Company, New York , 1959.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca , 1993.
Heine, H.: Histoautoradiographie der DNS in Trophoblastriesenzellen der Plazenta der Maus. Acta anat. 95:66-74, 1976.
Hemberger, M. and Cross, J.C.: Genes governing placental development. Trends Endocrinol. Metab. 12:162-168, 2001.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Folio 11, 58, 164, 165, 418. Springer Verlag, New York, 1967-1975.
Iwaki , T., Sandoval-Cooper, M.J., Paiva, M., Kobayashi, T., Ploplis, V.A. and Castellino, F.J.: Fibrinogen stabilizes placental-maternal attachment during embryonic development in the mouse. Amer. J. Pathol. 160:1021-1034, 2002.
Kaufman, M.H.: The Atlas of Mouse Development. Academic Press, London , 1992.
Laakkonen, J., Fisher, R.N. and Case, T.J.: Pneumocystosis in wild small mammals from California . J. Wildl. Dis. 37:408-412, 2001.
Luo, J., Sladek, R., Bader, J.-A., Matthyssen, A., Rossant, J. and Giguère, V.: Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-ß. Nature 388:778-782, 1997.
McAllister, C.T., Upton , S.J., Planz, J.V. and DeWalt, T.S.: New host and locality records of coccidian (Apicomplexa: Eimeriidae) from rodents in the southwestern and western United States . J. Parasitol. 77:1016-1019, 1991.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison , Wisconsin , 1973.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison , Wisconsin , 1973.
Nachman, M.W., Hoekstra, H.E. and D'Agostino, S.L.: The genetic basis of adaptive melanism in pocket mice. Proc. Natl. Acad. Sci. USA 100:5268-5273, 2003.
Nadler, C.F.: Chromosomal evolution in rodents. Pp. 277-309 in: Comparative Mammalian Cytogenetics, K. Benirschke, ed., Springer-Verlag , NY , 1969.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Patton, J.L.: Chromosome studies of certain pocket mice, genus Perognathus (Rodentia: heteromyidae). J. Mammal. 48:27-37, 1967.
Patton, J.L.: A complex system of chromosomal variation in the pocket mouse. Perognathus baleyi Merriam. Chromosoma 36:241-255, 1972.
Patton, J.L.: B-chromosome systems in the pocket mouse, Perognathus baileyi : meiosis and C-band studies. Chromosoma 60:1-14, 1977.
Ramsey, E.M.: The Placenta of Laboratory Animals and Man. Holt, Rinehart and Winston , New York , 1975.
Rossant, J. and Cross, J.C.: Placental development: Lessons from mouse mutants. Nature Reviews Genetics 2:538-548, 2001.
Rossant, J. and Croy, B.A.: Genetic identification of tissue of origin of cellular population within the mouse placenta. J. Embryol. Exp. Morph. 86:177-189, 1985.
Snow, M.H.L. and Ansell, J.D.: The chromosomes of giant trophoblast cells of the mouse. Proc. R. Soc. Lond. B.: 187:93-98, 1974.
Wahrman, J., Goitein, R. and Nevo, E.: Geographic variation of chromosome forms in Spalax, a subterranean mammal of restricted mobility. Pp. 30-48 in: Comparative Mammalian Cytogenetics, K. Benirschke, ed., Springer-Verlag , NY , 1969.