|
(Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.)
|
Sus scrofa domestica
Order: Artiodactyla
Family: Suidae
1) General Zoological Data
Suidae had their origin in Eurasia and distributed widely while speciating into significantly different phenotypes and behavioral repertoires. The Suiformes are presumed to be the most primitive of the artiodactyls. The Artiodactyla are considered to have formed three major groups: the Suiformes, Tylopoda and Pecora (Groves, 1981). Warthogs are believed to be perhaps the most recent derivatives of suidae. Bosma (1978) reviewed the reasons for placing Phacochoerus away from the other suidae because of their differential dental structures. Nevertheless, the similarity of chromosomes (v.i.) argues against this notion. She suggested a recent origin of this species. On the other hand, in his searching discussion of suid development, Thenius (1970) found justification of separating this most recent suid species; he placed the first ancestor into the middle of the Oligocene (+/- 28 MYA).
There are a very large number of special strains of domestic pigs. Some of the better-known varieties being the pot-bellied pig, miniature pig, etc. Males are generally larger. The longevity of various Sus species in captivity was given by Jones (1993). Maximal life span for a European wild pig was 21 years. Domestic pigs were not included in that survey. It is known, however, that external influences (leg weakness Jorgensen, 2000), age of first conception (Koketsu et al., 1999) and other factors affect longevity and productivity of swine and these may have important commercial ramifications (D'Allaire et al., 1992).
 |
Male and female pigs (from pigsong@korea.com) |
Hayssen et al. (1993) stated that "..the reproductive biology of domestic pigs is reviewed in supplements 33 and 40 of the Journal of Reproduction and Fertility published in 1985 and 1990 respectively". Pigs have a 21 day cycle with a period of heat 2-3 days long. Ovulation is spontaneous and pregnancy lasts 112 - 115 days (average 114 days). Domestic pigs have 3-16 young, much depending on the race (Asdell, 1946). Wild European pigs have on average 4 offspring. A much more comprehensive review of the reproductive physiology is provided by Geisert (1999). Ovulation and fertilization occur on the first day of estrus; blastocyst hatching occurs 6 days later; two days thereafter the blastocysts reach the uterus and elongate with initial adhesion of the trophoblast occurring in the endometrium 12 days after fertilization. On day 14, the allantois develops, and placental development commences 17 days after fertilization. Placental fusion of adjacent placentas is "very rare". But, when it happens, freemartins (blood chimerae) may develop if the litters are of opposite sex according to Hughes (1929). A high percentage of embryos are lost at different stages of gestation, estimated to be at least 30%. A detailed review of reproduction and embryonic development, as well as placentation may be found in the classic book on the pig by Patten (1947).
3) Implantation
Initial implantation is accomplished primarily by the yolk sac epithelium, which is then the dominant membrane, but it lasts only a short time. There is much uterine "milk" with absorption through this epithelium. By day 24 of the domestic pig pregnancy, the allantois attaches all around the periphery and the yolk sac shrinks (Ramsey, 1982, Mossman, 1987). Additional details of early phases of domestic pig reproduction are available from the review by Geisert (1999). The uterus is bicornuate, and multiple implantations occur in all pigs, first at a mesometrial site. The cord locates mesometrially.
4)
General Characterization of the Placenta
This is a diffuse epitheliochorial placenta with atrophy at the peripheral
tips, a feature that was already known when Turner (1876) gave a detailed
description of the pig placenta and uterus. He remarked on the ridge-like
pattern of the chorion and on the presence of circular regions that he
situated as corresponding to the "areolae" of the uterine mucosa
into which glands open. They are shown in good detail by Patten (1947),
Amoroso (1961), and by many other authors. Mossman (1987) remarked that
the placenta of swine is often much more gelatinous in its appearance
than that of other ungulates. This has certainly been my observation in
the placentas of wild pig species (see chapters on warthog and red river
hog). There is a large allantoic sac that is essential for nutrient transfer
from the maternal uterine secretion (Geisert, 1999). Since no invasion
occurs, much of the placenta/embryonic development depends on the "uterine
milk or embryotroph", endometrial secretions that are dominated by
progesterone and estrogen (Roberts & Bazer, 1998). The purple protein
"uteroferrin", often confused with non-specific acid and alkaline
phosphatases, is one of these important proteins. The acid phosphatase
associates with uteroferrin, while the alkaline phosphatase has been localized
to the microvasculature of the endometrium and is distinct from uteroferrin
(Firth et al., 1986). The trophoblast also has a high content of acid
phosphatase; other enzymes interacting between endometrium and trophoblast
are considered in the study by Skolek-Winnisch et al. (1985). The importance
of the hist(i)otroph is further demonstrated by the protein in the dome-shaped
formations over the openings of uterine glands, the areolae, which were
studied ultrastructurally by Friess et al. (1981).
An excellent and very detailed account of the pig placenta comes from
Amoroso (1961). This very readable presentation also gives adequate reference
to all former publications on pig placentation. Among many topics, he
addressed the question as to whether the trophoblast and endometrial epithelium
are separated by "milk" and find this to be highly unlikely.
His photographs also show direct apposition. Binucleated trophoblast is
not found, and there is normally no pigmentation.
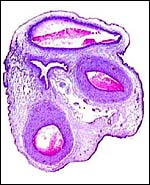
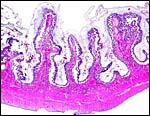 |
Implanted pig placenta early in gestation. The interdigitation of villi and endometrium is apparent. |
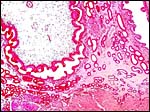 |
Higher magnification of young implanted pig placenta with endometrial gland and myometrium below. |
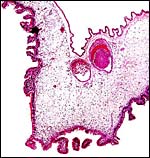
The placenta of all suidae is a typical diffuse epitheliochorial organ without invasion of the endometrium (Amoroso, 1961; Mossman1987; Macdonald & Bosma, 1985). The latter authors have compared a number of suid placentas and pregnant uteri, including those of the warthog. They described the chorion as being rippled and having indentations of trophoblast by capillaries. The single-layered trophoblast interdigitates with endometrial epithelial folds. Mossman (1987), and Macdonald & Bosma (1985) both described the arcades and areolae and the importance of uteroferrin transfer to the fetus. This progesterone-induced glycoprotein ("pig purple acid phosphatase" - Twitchett et al., 2002; Klabunde et al., 1995; Nuttleman & Roberts, 1990) from the endometrium has been studied extensively (Renegar et al., 1982) and is apparently transferred to the fetus through the allantoic fluid (Buhi et al., 1983; Geisert, 1999; further discussion below). Administration of iron and tetrahydrofolate had no effect on fetal iron concentration (Vallet et al., 2001). Binucleated cells as known from ungulates do not occur. Ludwig (1968) compared numerous species' interaction of trophoblast-endometrial surface relations and grouped the Suina with the placentas that have an "enteroid" function.
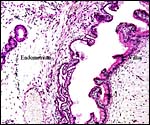 |
Endometrial gland at left, villus at right. |
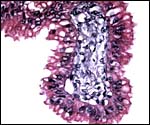 |
The trophoblast in this young placenta is single-layered and cylindrical. |
The umbilical cord contains three blood vessels and a widely patent allantoic duct. In this immature specimen, few smaller allantoic vessels are found in the cord; later in gestation more are formed. Also, because of the immaturity of this specimen, there is no squamous metaplasia which, again, develops later. I cannot find any detailed reference in the literature on the length of pig umbilical cords.
 |
Immature pig umbilical cord with three vessels, vein on top, allantoic duct at left. |
I am not aware of any detailed description but surely studies must have been done.
8)
Extraplacental membranes
There is a large and highly vascularized allantoic sac that is lined by
cuboidal to cylindrical epithelium. Much allantoic fluid collects early
in gestation, and much work has been done to understand its role for protein
transfer to the fetus (Geisert, 1999), especially of the uteroferrin.
The allantoic sac is very large in early gestation and, as pregnancy advances,
the amnionic sac enlarges more prominently and ultimately contains as
much as 200 ml of fluid. Amoroso (1961) also stated that the allanto-amnion
becomes vascularized during the course of pregnancy.
 |
Free allanto-amnion with amnion above and vascularized allantoic membrane below. |
No endometrial invasion by trophoblast occurs.
10)
Endometrium
The endometrium has no caruncles and consists mostly of glands whose description
during gestation has been detailed by Heuser (1927). Amoroso (1961) further
described them in some detail and stated that they secrete iron; "the
glandular orifices are covered by domes of trophoblastic cells - the
areolae - which absorb the secretion (uterine milk) and transmit
it as nutriment for the developing embryo by the allantoic vessels".
The endometrium of pigs plays a major role in secreting various nutrients,
and transfer is at least partially taking place through the allantoic
fluid (Geisert, 1999). Among these nutrients, uteroferrin, an iron-transporting
acid phosphatase, is an important aspect of fetal iron supply. It has
been studied extensively (see also above) and arises from the areolar
region of the endometrium (Dantzer & Nielsen, 1984). The role of endometrial
cells was studied in vitro by Davis & Blair (1993) who
investigated individual components of the endometrial epithelial cells
in their secretory roles of uteroferrin and prostaglandin production.
The localization to the non-ciliated endometrial glandular epithelial
cells "overlaying the areolae" and the adjacent trophoblast
was accomplished by Raub et al. (1985), using immunocolloidal gold-labels.
The events of post partum discharge and uterine involution (macroscopic
and histologic) were discussed extensively by McEntee (1990).
11) Various features
There is no subplacenta. The pig placenta is especially "gelatinous",
but we do not know the reason for this feature, nor do we understand the
nature of the content of this gelatinous material.
12)
Endocrinology
Austin & Short (1972) wrote: "In this species, the nature of
the luteotrophic complex changes during gestation. Early in pregnancy,
the corpora lutea are relatively independent of pituitary control and
their survival depends on the ability of the conceptus to neutralize the
uterine lytic factor. Later, up to about day 40, LH is the predominant
luteotrophic factor, but thereafter prolactin becomes increasingly important.
Oestrogens also may play a part in the maintenance of the corpus luteum,
possibly acting indirectly to stimulate pituitary prolactin secretion;
the placenta of the pig produces appreciable amounts of oestrogen in late
pregnancy." The changes in hormone production during pregnancy are
graphically represented by Geisert (1999). Involution of the corpora lutea
during pregnancy is prevented by the conceptus' secretion of PGF2a. Pregnancy
is much dependent upon the secretion of on the timely production of estrogens
and is readily disrupted by environmental; agents (see Tarleton et al.,
2003). Bazer & Roberts (1983) related that pig blastocysts begin estrogen
production on day 11 of pregnancy, thus inhibiting the otherwise prostaglandin-induced
luteolysis. The glandular epithelium is also responsible for the secretion
of a variety of endometrial proteins. Mossman & Duke (1973) described
the ovaries of various pigs and found them to be enclosed in a partial
bursa, with a wide opening. Fewer corpora lutea (4) were found in wild
European pigs than in domestic pigs.
13)
Genetics
The domestic pig has 38 chromosomes, but its wild ancestors may have 38
or 36 chromosomes, with fertile hybrids possessing 37 (McFee et al., 1966;
Gropp et al., 1969). Robertsonian fusions have generally played an important
role in suid speciation (Bosma et al., 1991). Several chromosomal errors
have been described and are listed in the pathology section and are also
added in the appendices to Hsu & Benirschke (1967). A variety of Sus
species have hybridized (Gray, 1972). The gene for uteroferrin secretion
has now been localized to chromosome # 2 (Yasue et al., 1995), and it
has been further characterized by Vallet & Fahrenkrug (2000).
 |
Karyotypes of male and female domestic pigs, 2n=38. |
 |
Karyotypes of male and female domestic pigs, 2n=38. |
Immunological studies have been mostly related to infectious diseases occurring in swine or of diseases that are thought to be transmitted through them. Recognition has also been of importance because of the transplants of pig cardiac valves. Thus, foot-and-mouth disease has been identified from swine in abattoirs in England (Alexandersen et al., 2003). Immunohistochemistry is being employed for porcine reproductive and respiratory syndrome virus disease (Yaeger, 2002), and this disease has been experimentally studied, among others by Shibata et al. (2003). These papers will give access to numerous other immunological studies.
15)
Pathological features
A variety of chromosomal errors have been identified in domestic pigs.
In a boar with a statistically lower litter sizes, Locniskar et al. (1976)
found a reciprocal translocation (1p-;6q+). Madan et al. (1978) identified
a translocation t(6p;15q-) in a boar with "intersex" genitalia
(bilateral ovotestes) whose ovarian portions had ovulated. Booth &
Polge (1976) reported chimeric pigs. Hancock & Daker (1981) reported
on a sterile 39,XXY boar. In pigs with anal atresia, Vogt (1967) found
no chromosomal errors in lymphocyte cultures. Henricson & Bäckström
(1964) did meiotic studies on normal and subfertile boars, without finding
correlations. McFeely (1967) reported on studies of early blastocysts
in which he found numerous chromosomal errors, such as triploidy (XXX
& XXY), tetraploidy and chimeras. It is possible that some of these
abnormal embryos begin implantation but degenerate and leave the spaces
in the uterus noted in earlier studies (33% according to Hanley, 1961).
Hydrometra, intersexes, and some other reproductive pathologic features
were reviewed by McEntee (1990).
Numerous infectious diseases occur in pigs and are discussed in texts
of veterinary pathology, e.g. Smith et al. (1972). Thus, brucellosis is
more fulminant in this species and may lead to chronic placentitis. Foot-and-mouth
disease became a problem in England after infection of a group of pigs
in 2001 (Alexandersen et al., 2003). Swine suffer many other diseases
such as leg weakness and, importantly, an infection with the reproductive
and respiratory syndrome virus (Yaeger, 2002).
16)
Physiologic data
Some physiologic data are discussed by Asdell (1946), including the migration
of eggs in the uterus which is also discussed by Geisert (1999). As has
already been alluded to above, much work has been undertaken to clarify
the role of uteroferrin (UF) in iron transport to the fetus' hematopoietic
organs. A quantitative relation exists of UF and hematopoiesis (Ducsay
et al., 1986). Iron treatment during pregnancy had no significant effect
on neonatal hematologic values (Ducsay et al., 1984). These investigations
have been extended to encompass neonatal pigs by injection of uteroferrin
and these identified its role in stimulating hematopoiesis (Laurenz et
al., 1997; Bazer et al., 1991). In utero measurements by Vallet et al.
(1996) indicated a relationship to protection against lipid peroxidation
of various components of this transfer system. Hematopoietic binding sites
were investigated by Michel et al. (1992) who found them to be invariably
present and perhaps unrelated to neonatal anemia. Doi et al. (1986) concluded
that direct iron transfer to apotransferrin is an unlikely physiological
role of UF.
17)
Other resources
There is a huge literature on domestic pigs. Cells are stored in a variety
of personal cell banks and cells of domestic pigs and pot-bellied pigs
are also available from the cell bank at the San Diego Zoo's "CRES",
by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
Domestic pigs have first been cloned in 2000. Since "absorption"
of fetuses is relatively common in domestic pigs (up to 30% or perhaps
even more), it would be of interest to know whether these "resorbed"
fetuses have similar chromosomal errors as those that have been described
for blastocysts by McFeely (1967). This is of special interest because
a variety of chromosomal errors have also been identified in adult domestic
pigs.- There is a deficiency in our knowledge of the length of pig umbilical
cords.
Acknowledgement
The photograph of the sow and boar at the beginning of this chapter comes
from the internet: pigsong@korea.com
References
Alexandersen, S., Kitching, R.P., Mansley, I.M. and Donaldson, A.I.: Clinical
and laboratory investigations of five outbreaks of foot-and-mouth disease
during the 2001 epidemic in the United Kingdom. Vet. Rec. 152:489-496,
2003.
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall's Physiology of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown & Co. Boston, 1961.
Asdell, S.A.: Patterns of Mammalian Reproduction. Comstock Publishing, Co., Inc. Ithaca, NY 1946.Austin, C.R. and Short, R.V.: Reproduction in Mammals. 3 Hormones in Reproduction. Cambridge University Press, 1972.
Bazer, F.W. and Roberts, R.M.: Biochemical aspects of conceptus-endometrial interactions. J. Exp. Zool. 228:373-383, 1983.
Bazer, F.W., Worthington-White, D., Fliss, M.F. and Gross, S.: Uteroferrin: a progesterone-induced hematopoietic growth factor of uterine origin. Exp. Hematol. 19:910-915, 1991.
Bosma, A.A.: The chromosomal G-banding pattern in the wart hog, Phaecochoerus aethiopicus (Suidae, Mammalia) and its implications for the systematic position of the species. Genetica 49:15-19, 1978.
Bosma, AA., deHaan, N.A. and MacDonald, A.A.: The current status of cytogenetics of the Suidae: A review. Bongo 18:258-272, 1991.
Buhi, W.C., Ducsay, C.A., Bartol, F.F., Bazer, F.W. and Roberts, R.M.: A function of the allantoic sac in the metabolism of Uteroferrin and maternal iron by the fetal pig. Placenta 4: Spec No. 455-469, 1983.
D'Allaire, S., Leman, A.D. and Drolet, R.: Optimizing longevity in sows and boars. Vet. Clin. North Amer. Food Anim. Pract. 8:545-557, 1992.
Dantzer, V. and Nielsen, M.H.: Intracellular pathways of native iron in the maternal part of the porcine placenta. Europ. J. Cell Biol. 34:103-109, 1984.
David, D.L. and Blair, R.M.: Studies of uterine secretions and products of primary cultures of endometrial cells in pigs. J. Reprod. Fertil. Suppl. 48:143-155, 1993.
Doi, K., Antanaitis, B.C. and Aisen, P.: Absence of iron transfer from uteroferrin to transferring. J. Biol. Chem. 261:14936-14938, 1986.
Ducsay, C.A., Buhi, W.C., Bazer, F.W., Roberts, R.M. and Combs, G.E.: Role of Uteroferrin in placental iron transport: effect of maternal iron treatment on fetal iron and Uteroferrin content and neonatal hemoglobin. J. Anim. Sci. 59:1303-1308, 1984.
Ducsay, C.A., Buhi, W.C., Bazer, F.W. and Roberts, R.M.: Role of Uteroferrin in placental iron transport in swine: relationship between Uteroferrin levels and iron deposition in the conceptus during gestation. J. Anim. Sci. 62:706-716, 1986.
Firth, J.A., Sibley, C.P. and Ward, B.S.: Histochemical localization of phosphatases in the pig placenta: I. Non-specific phosphatases and their relation to uteroferrin. Placenta 7:17-25, 1986.
Friess, A.E., Sinowatz, F., Skolek-Winnisch, R. and Trautner, W.: The placenta of the pig. II. The ultrastructure of the areolae. Anat. Embryol. 163:43-53, 1981.
Geisert, R.D.: Pigs. Pp. 792-799 in Vol. III of, Encyclopedia of Reproduction, E. Knobil and J.D. Neill, eds., Academic Press, San Diego, 1999.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Gropp A, Giers D, and Tettenborn U.:Das Chromosomenkomplement des Wildschweins (Sus scrofa). Experientia 25:778. 1969.
Groves, C.: Ancestors for the pigs: taxonomy and phylogeny of the genus Sus. Technical Bulletin No. 3. Department of Prehistory, Research School of Pacific Studies, Australian National University, 1981.
Hancock, J.L. and Daker, M.G.: Testicular Hypoplasia in a boar with abnormal sex chromosome constitution (39 XXY). J. Reprod. Fertil. 61:395-397, 1981.
Hanley, S.: Prenatal mortality in farm animals. J. Reprod. Fertil. 2:182, 1961.
Hansen, K.M.: Identification of the chromosomes of the domestic pig (Sus scrofa domestica). An identification key and a landmark system. Ann. Genet. Sel. Anim. 9:517-526, 1977.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Henricson, B. and Bäckström, L.: A systematic study of the meiotic divisions in normal and subfertile or sterile boars and bulls. J. Reprod. Fertil. 7:53-64, 1964.
Heuser, C.H.: A stud of the implantation of the ovum of the pig from the stage of the bilaminar blastocyst to the completion of the fetal membranes. Contrib. Embryol. Carnegie Institution 19:229-243, 1927.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Vol. 1, Folio 38 Springer-Verlag, New York. 1967.
Hughes,
W.: The freemartin condition is swine. Anat. Rec.41:213-245, 1929.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk.
32:159-169, 1993.
Jorgensen, B.: Longevity of breeding sows in relation to leg weakness symptoms at six months of age. Acta Vet. Scand. 41:105-121, 2000.
Klabunde, T., Strater, N., Krebs, B. and Witzel, H.: Structural relationship between the mammalian Fe(III)-Fe(II) and the Fe(III)-Zn(II) plant purple acid phosphatases. FEBS Lett. 367:56-60, 1995.
Koketsu, Y., Takahashi, H. and Akachi, K.: Longevity, lifetime pig production and productivity, and age at first conception in a cohort of gilts observed over six years on commercial farms. J. Vet. Med. Sci. 61:1001-1005, 1999.
Laurenz, J.C., Hadjisavas, M., Schuster, D. and Bazer, F.W.: The effect of uteroferrin and recombinant GM-CSF on hematopoietic parameters in normal female pigs (Sus scrofa). Comp. Biochem. Physiol. B Biochem Mol. Biol. 118:579-586, 1997.
Locniskar, F., Gustavsson, I., Hageltorn, M. and Zech, L.: Cytological origin and point of exchange of a reciprocal chromosome translocation (1p-;6q+) in the domestic pig. Hereditas 83:272-275, 1976.
Ludwig, K.S.: Zur vergleichenden Histologie des Allantochorions. Rev. Suisse Zool. 75:819-831, 1968.
Macdonald, A.A. and Bosma, A.A.: Notes on placentation in the Suina. Placenta 6:83-91, 1985.
MacEntee, K.: Reproductive Pathology of Domestic Mammals. Academic Press, Inc. San Diego, 1990.
Madan, K., Ford, C.E. and Polge, C.: A reciprocal translocation, t(6p+;14q-), in the pig. J. Reprod. Fertil. 53:395-398, 1978.
McFee, A.F., Banner, M.W. and Rary, J.M.: Variation in chromosome number among European wild pigs. Cytogenetics. 5:75-81, 1966.
McFeely, R.A.: Chromosome abnormalities in early embryos of the pig. J. Reprod. Fertil. 13:579-581, 1967.
Michel, F.J., Fliss, M.F., Bazer, F.W. and Simmen, R.C.: Characterization and developmental expression of biding sites for the transplacental iron transport protein, uteroferrin, in fetal hematopoietic tissues. Biol. Neonat. 61:82-91, 1992.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press1973).
Nuttleman, P.R. and Roberts, R.M.: Transfer of iron from Uteroferrin (purple acid phosphatase) to transferring related to acid phosphatase activity. J. Biol. Chem. 265:12192-12199, 1990.
Patten, B.M.: The Embryology of the Pig. 2nd ed. The Blakiston Co. Philadelphia, 1931 (reprinted 1947).
Ramsey, E. M.: The Placenta. Human and Animal. Praeger, N.Y., 1982.
Raub, T.J., Bazer, F.W. and Roberts, R.M.: Localization of the iron transport glycoprotein, Uteroferrin, in the porcine endometrium and placentae by using immunocolloidal gold. Anat. Embryol. 171:253-258, 1985.
Renegar, R.H., Bazer, F.W. and Roberts, R.M.: Placental transport and distribution of Uteroferrin in the fetal pig. Biol. Reprod. 27:1247-1260, 1982.
Roberts, R.M. and Bazer, F.W.: The functions of uterine secretions. J. Reprod. Fertil. 82:875-892, 1988.
Shibata, I., Yazawa, S., Ono, M. and Okuda, Y.: Experimental dual infection of specific pathogen-free pigs with porcine reproductive and respiratory syndrome virus and pseudorabies virus. J. Vet. Med. B Inf. Dis. Vet. Public Health 50:14-19, 2003.
Skolek-Winnisch, R., Lipp, W., Sinowatz, F. and Friess, A.E.: Enzymhistochemische Untersuchungen an der Schweineplacenta. II. Histotopik von Enzymen in den areolären Placentaepithelien. Acta Histochem. 76:131-143, 1985.
Smith, H.A., Jones, T.C. and Hunt, R.D.: Veterinary Pathology. Lea & Febiger, Philadelphia, 1972.
Tarleton, B.J., Braden, T.D., Wiley, A.A. and Bartol, F.F.: Estrogen-induced disruption of neonatal porcine uterine development alters adult uterine function. Biol. Reprod. 68:1387-1393, 2003.
Thenius, E.: Zur Evolution und Verbreitungsgeschichte der Suidae (Artiodactyla, Mammalia). Z. Säugetierk. 35:321-342, 1970.
Turner, W. The structure of the diffused, the polycotyledonary and the zonary forms of placenta. J. Anat. Physiol. 10:127-177, 1876.
Twitchett, M.B., Schenk, G., Aquino, M.A., Yiu, D.T., Lau, T.C. and Sykes, A.G.: Reactivity of M(II) metal-substituted derivatives of pig purple acid phosphatase (Uteroferrin) with phosphate. Inorg. Chem. 41:5787-5794, 2002.
Vallet, J.L., Christenson, R.K. and McGuire, W.J.: Association between uteroferrin, retinal-binding protein, and transferring within the uterine and conceptus compartments during pregnancy in swine. Biol. Reprod. 55:1172-1178, 1996.
Vallet, J.L., Christenson, R.K., Klemcke, H.G. and Pearson, P.L.: Intravenous infusion of iron and tetrahydrofolate does not influence intrauterine Uteroferrin and secreted folate-binding protein content in swine. J. Anim. Sci. 79:188-192, 2001.
Vallet, J.L. and Fahrenkrug, S.C.: DNA Cell Biol. 19:689-696, 2000.
Vogt, D.W.: Chromosome condition of two atresia ani pigs. J. Anim. Sci. 26:1002-1004, 1967.
Yaeger, M.J.: The diagnostic sensitivity of immunohistochemistry for the detection of porcine reproductive and respiratory syndrome virus in the lung of vaccinated and unvaccinated swine. J. Vet. Diagn. Invest. 14:15-19, 2002.
Yasue, H., Kusumoto, H. and Mikami, H.: Assignment of the uteroferrin gene (ACP5) to swine chromosome 2q12->q21 by fluorescence in situ hybridization. Cytogenet. Cell Genet. 71:249-252, 1995.
