| |
13) Genetics
The taguá has 20 chromosomes, in contrast to the other two species.
They have, respectively, 26 and 30 elements. In the genetic study of mtDNA,
Theimer and Keim (1988) suggested that the invasion of peccary ancestors
to South America in the Pliocene led to their segregation into separate
clades (see also Marshall, 1988). Although these species all occur sympatrically
in the Chaco, hybridization with the other species has not been recorded.
The putative evolutionary chromosomal rearrangements have been described
for the three tayassuid species, including banding homologies (Benirschke
et al., 1985; Benirschke & Kumamoto, 1989). The evolution of tayassuids
has been considered in some detail by Wetzel (1977) and it was also the
topic of Wallace (1988). A studbook is being maintained of the captive population.
In a recent cytogenetic study by Bosma et al. (2004), considerable chromosomal differences were found in the finer details of chromosome morphology between the collared and white-lipped peccaries. It contrasts with the more conservative arrangement of the suid chromosome morphology. Possible explanations were speculated upon and await further study.
Gongora & Moran (2005) have further examined nuclear and mitochondrial genes of all three species. They found greater mitochondrial gene divergence in collared peccaries than among the other two species, which are more closely related to each other than to collared peccaries. In addition, these authors reconsidered the question as to whether divergence originated before or after invasion of peccaries into South America after the landbridge was established. But final resolution of this question still awaits additional specimen study.
14)
Immunology
There have been no reported studies.
15)
Pathological features
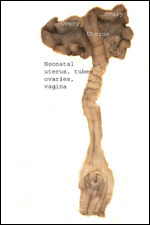
The neonatal mortality is high in captive situations, but is it unknown
for animals in the wild. Inadequate nutrition, sand imbibition, and pneumonia
are the principal causes. Parasitism is very low in wild specimens (microfilaria,
Balantidium sp.) and consists mostly of ectoparasites (Amblyoma sp.).
I have seen one case of an apparently viral pneumonia that closely resembled
Jaagsiekte of ruminants. A questionable spontaneous intestinal perforation
has been observed. Tooth abnormalities and traumatic lesions occur, and
much inhaled dust is found in adult animals' lungs. A partial posterior
duplication suggested that conjoined twinning, perhaps monozygotic twinning,
occurs. Placental infections have not been observed. Infections with Chlamydia
psittaci was seen in a zoo because of the exposure to adjacent other artiodactyl
animals that harbored the agent.
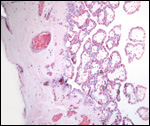
Older taguá often suffer chronic renal disease with renal and bladder
calculi. They are comprised of oxalates. This is probably attributable
to the preferred diet of cactus (Opuntia sp.). This putative relation
to diet is in urgent need of being proven. Occasionally, taguá
have had infections in the large scent glands located in the central portion
of their dorsal sacral region. Much oily fluid can be secreted from this
gland; the glandular secretions of the three species also have very different
odors. It is of interest to learn what the fetal/maternal ovarian interstitial
cell produces. Studies by incubating this tissue are needed.
16)
Physiological data
No studies have been reported.
17)
Other resources
Cell strains of many specimens are contained in the "Frozen Zoo"
of "CRES" at the San Diego Zoo.
18) Other features of interest
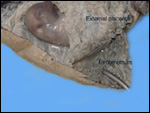
The similarity of placentation to that of Suidae is striking. Please see
the chapter on "Red River Hog". An extensive bibliography on
peccaries can be downloaded from a section of CRES at the web site of
the San Diego Zoo (www.sandiegozoo.org).
References
Benirschke, K.: Anatomic studies on pregnant giant peccaries (Catagonus
wagneri). Zool. Garten 70:201-210, 2000.
Benirschke,
K., Byrd, M. and Meritt, D.: New observations on the Chacoan peccary,
Catagonus wagneri. Pp. 341-347, In. 32nd International Symposium
on Erkrankungen der Zootiere (Eskilstuna, 1990). Akademie-Verlag, Berlin.
Benirschke,
K., Gonzalez, S., Gould, G., Byrd, M. and Kumamoto, A.: Observations on
the Chacoan peccary (Catagonus wagneri). Pp. 391-397, In, 28th
International Symposium on Erkrankungen der Zootiere (Rostock, 1986).
Akademie-Verlag, Berlin.
Benirschke,
K., Hager, D.A. and Edwards, D.K.: Observations on neonatal mortality
of the Chacoan peccary Catagonus wagneri. Vet. Path. 37:532-534,
1995.
Benirschke, K., Kumamoto, A.T. and Meritt, D.A.: Chromosomes of the Chacoan
peccary, Catagonus wagneri (Rusconi). J. Hered. 76:95-98. 1985.
Benirschke,
K. and Kumamoto, A.T.: Further studies on the chromosomes of three species
of peccary. Adv. Neotrop. Mammal. 309-316, 1989.
Benirschke,
K., Low, R.J. and Byrd, M.: Further observations on the causes of death
in the Chacoan peccary, Catagonus wagneri. Pp. 71-78, In 31st International
Symposium on Erkrankungen der Zootiere (Dortmund, 1989). Akademie-Verlag,
Berlin.
Brooks,
D.M.: Reproductive behaviour and development of the young of the chacoan
peccary (Catagonus wagneri, Rusconi, 1930) in the Paraguayan Chaco.
Z. Säugetierk. 57:316-317, 1992.
Bosma, A.A., de Haan, N.A., Arkesteijn, G.J.A., Yang, F., Yerle, M. and Zijlstra, C.: Comparative chromosome painting between the domestic pig (Sus scrofa) and two species of peccary, the collared peccary (Tayassu tajacu) and the white-lipped peccary (T. pecari): a phylogenetic perspective. Cytogenet. Genome Res. 195:115-121, 2004.
Byrd,
M.L., Benirschke, K. and Gould, G..C.: Establishment of the first captive
group of the Chaco peccary, Catagonus wagneri. Zool. Garten 5/6:265-274,
1988.
Cell
strains from: www.sandiegozoo.org
(CRES section).
Gongora, J. and Moran, C.: Nuclear and mitochondrial evolutionary analyses of Collared, White-lipped, and Chacoan peccaries (Tayassuidae). Molec. Phylogenet. Evol. 34:181-189, 2005.
Handen,
C.E., Unger, J. and Meritt, D.: Current status of the taguá (Catagonus
wagneri) in Paraguay. Zool. Garten 64:329-337, 1994.
Macdonald,
A.A. and Bosma, A.A.: Notes on placentation in the Suina. Placenta 6:83-92,
1985.
Marshall,
L.G.: Land Mammals and the Great American Interchange. Amer. Scientist
76:380-386, 1988.
Mayer,
J.J. and Brandt, P.N.: Identity, distribution, and natural history of
the peccaries, Tayassuidae. In, Mammalian Biology in South America (M.A.
Mares & H.H. Genoways, eds.) Special Publication Series Pymatuning
Lab. Ecol., University of Pittsburgh, pp. 433-454, 1982.
Mayer, J.J. and Wetzel, R.M.: Catagonus wagneri. In, Mammalian Species
# 259, pp. 1-5, 1986. Amer. Soc. Mammalogists.
Miglino,
M.A., Santos, T.C. and Dantzer, V.: The collared peccary (Tayassu tajacu
Linnaeus, 1795) and white-lipped peccary (Tayassu pecari Link,
1795) placenta. Abstract 80 in, Placenta 22(7):A28, p80, 2001.
Oliver,
W.L.R.: Pigs, Peccaries, and Hippos. IUCN, Gland, Switzerland, 1993.
Olrog,
C.C., Ojeda, R.A. and Barquez, R.M.: Catagonus wagneri (Rusconi)
en el Noreste Argentino. Neotropica 22:53-56, 1976.
Santos, T.C., Dantzer, V., Jones, C.J.P., Oliveira, M.F. and Miglino, M.A.: Macroscopic and microscopic aspects of collared peccary and white-lipped peccary placenta. Placenta. 27:244-257, 2006.
Sowls,
L.K.: The Peccaries. (Chapter 10, the Chacoan Peccary). Univ. Arizona
Press, Tucson, AZ, 985.
Taber,
A.: Monographie des Chaco-Pekaris (Catagonus wagneri). Bongo 18:135-150,
1990.
Theimer,
T.C. and Keim, P.: Phylogenetic relationships of peccaries based on mitochondrial
cytochrome B DNA sequences. J. Mammal. 79:566-572, 1998.
Wetzel,
R.M. and Crespo, J.A.: Existencia de una tercera especie de pecari, Fam.
"Tayassuidae, Mammalia", en Argentina. Rev. Museo Argentino
de Sciencias Naturales 12:25-26, 1975.
Wetzel,
R.M., Dubos, R.E., Martin, R.L. and Myers, P.: Catagonus, an "extinct"
peccary, alive in Paraguay. Science 189:379-381, 1975.
Wetzel,
R.M.: The Chacoan Peccary Catagonus wagneri (Rusconi). Bull. Carnegie
Museum Natural History, Pittsburgh, # 3, 1-36, 1977.
Yahnke,
C.J., Unger, J., Lohr, B., Meritt, D.A. and Heuschele, W.: Age specific
fecundity, litter size, and sex ratio in the Chacoan peccary (Catagonus
wagneri). Zoo Biol. 16:301-307, 1997.
|