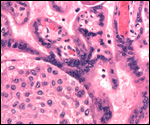
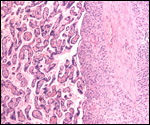
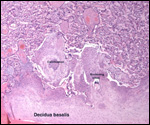
| |
16) Physiological data
General laboratory data (hematology, etc.) became available from the studies
by Sly et al. (1978). Kessler et al. (1983) studied hematologic values in
the free-ranging Puerto Rico animals. Paterson (1975) showed that a wide
variation of biochemical parameters exists in patas monkeys. Thermoregulation
was studied by Gisolfi et al. (1983), which included some heart rate measurements
as well.
17) Other resources
There is a large number (+/- 150) of free-ranging patas monkeys on Puerto
Rico. They have escaped from the former breeding colony and are considered
to be a "pest" now. Other colonies are held in a few laboratories
referred to by Sly et al. (1983).
Cell
lines are available from CRES at the Zoological Society at San Diego.
18)
Other relevant features
Mattison & King (1983) have proposed this animal and other cercopithecids
for the experimental study of fetoscopy and fetal blood sampling. When
pregnant patas monkeys were given zidovudine (AZT - used for treatment
of AIDS) in doses equivalent to human therapy, the fetuses (heart and
muscle) and their placentas showed a dose-dependent damage of their mtDNA
(Gerschenson & Poirier, 2000).
Twinning is not very common and it is rarely successful. Sly et al. (1983)
reported that 4 sets of twins were observed among 501 gestations. Of these,
one set aborted at midgestation, one had a mummified fetus with a liveborn
infant, one liveborn had a stillborn twin, and two twins were stillborn.
Sex and zygosity were not mentioned.
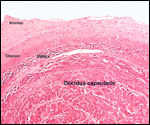
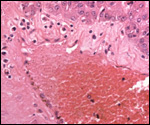
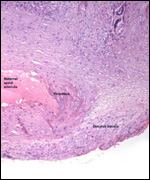
Conaway (1969) described adrenal rests at the ovarian hilus, a frequent
finding in many other species and also occurring near testes. The interesting
aspect of these rests is that they never have any medullary tissue.
In view of the large proportion of "X-cells" in Patas monkey
placentas it would be useful to search for major basic protein MBP in
them, ascertain whether the human antibodies label them and perhaps reexamine
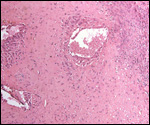
the "choriocarcinomas" described earlier.
References
Atkinson, L.E., Hotchkiss, J., Fritz, G.R., Surve, A.H., Neill, J.D. and
Knobil, E.: Circulating levels of steroids and chorionic gonadotropin
during pregnancy in the rhesus monkey, with special attention to the rescue
of the corpus luteum in early pregnancy. Biol. Reprod. 12:335-345, 1975.
Baylet,
R. and Grattepanche, H.: Sur les chromosomes des cercopithecidae Papio
papio, Macaca mulatta, Cercopithecus aethiops, Erythrocebus
patas. C.R. Soc. Biol. 158:1382, 1964.
Benirschke,
K. and Kaufmann, P.: The Pathology of the Human Placenta. 4th edition.
Springer-Verlag, NY, 2000.
Benirschke,
K. and Miller, C.J.: Anatomical and functional differences in the placenta
of primates. Biol. Reprod. 26:29-53, 1982.
Bibollet-Ruche,
F., Galat-Luong, A., Cuny, G., Sarni-Manchado, P., Galat, G., Durand,
J.P., Pourrut, X. and Veas, F.: Simian immunodeficiency virus infection
in a patas monkey (Erythrocebus patas): Evidence for cross-species
transmission from African green monkeys (Cercopithecus aethiops sabaeus)
in the wild. J. Gen. Virol. 77:773-781,
1996.
Cell
strains: CRES at http://www.sandiegozoo.org/conservation/cres_home.html.
Please direct your inquiries to Dr. Oliver Ryder (oryder@ucsd.edu).
Chabra,
S.K., Reed, C.D., Anderson, L.M. and Shiao, Y.H.: Comparison of the polymorphic
regions of the cytochrome P45 CYP2E1 gene of humans and patas and cynomolgus
monkeys. Carcinogenesis 20:1031-1034, 1999.
Conaway,
C.H.: Adrenal cortical rests of the ovarian hilus of the patas monkey.
Folia Primatol. 11:175-180, 1969.
Espana,
C., Gajdusek, D.C., Gibbs, C.J. Jr. and Lock, K.: Transmission of Creutzfeldt-Jakob
disease to the patas monkey (Erythrocebus patas) with cytopathological
changes in in vitro cultivated brain cells. Intervirology 6:150-155, 1975-1976.
Gerschenson,
M. and Poirier, M.C.: Fetal patas monkeys sustain mitochondrial toxicity
as a result of in utero zidovudine exposure. Ann. N.Y. Acad. Sci. 918:269-281,
200o.
Gille,
J.H., Moore, D.J. and Sedgwick, C.J.: Placental infarction: A sign of
preeclampsia in a patas monkey (Erythrocebus patas). Lab. Anim.
Sci. 27:120-121, 1977.
Gisolfi,
C.V., Wall, P.T. and Mitchell, W.R.: Thermoregulatory responses to central
injections of excess calcium in monkeys. Amer. J. Physiol. 245:R76-82,
1983.
Gonzalez-Martinez,
J.: The ecology of the introduced monkey (Erythrocebus patas) population
of southwestern Puerto Rico. Amer. J. Primatol. 45:351-365, 1998.
Goswell,
M.J. and Gartlan, J.S.: Pregnancy, birth and early infant behaviour in
the captive patas monkey Erythrocebus patas. Folia Primatol. 3:189-200,
1965.
Gotch,
A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset,
1979.
Gray,
A.P.: Mammalian Hybrids. Second edition. A Check-List with Bibliography.
Commonwealth Agricultural Bureaux, Farnham Royal, Slough, UK, 1972.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, 1983.
Hsu,
T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes, Vol. 8, Folio
399, 1974. Springer-Verlag, NY.
Kaplan,
J.R., Anthony, M. and Wood, J.: Domestic breeding of patas monkeys (Erythrocebus
patas). Lab. Anim. Sci. 31:409-412, 1981.
Kessler,
M.J., Phoebus, E.C., Rawlins, R.G., Turnquist, J.E. and London, W.T.:
Blood values of free-ranging patas monkeys (Erythrocebus patas).
J. Med. Primatol. 12:209-217, 1983.
Kuyl,
A.C.v.d., Kuiken, C.L., Dekker, J.T. and Goudsmit, J.: Phylogeny of African
monkeys based upon mitochondrial 12S rRNA sequences. J. Molec. Evol. 40:173-180,
1995.
Leakey,
L.S.: Presumed super-foetation in an Erythrocebus patas monkey.
Nature 223:754, 1969.
Nowak,
R.M. and Paradiso, J.L.: Walker's Mammals of the World, Vol. II. 4th edition.
The Johns Hopkins University Press, Baltimore, 1983.
Loomis,
M.R., O'Neill, T., Bush, M. and Montali, R.J.: Fatal herpesvirus infection
in patas monkeys and a black and white colobus monkey. J. Amer. Vet. Med.
Assoc. 179:1236-1239, 1981.
Lucotte,
G. and Dandieu, S.: Electrophoretic polymorphism in Erythrocebus patas.
(In French). Folia Primatol. 40:197-204, 1983.
Mahley,
R.W., Johnson, D.K., Pucak, G.J. and Fry, D.L.: Atherosclerosis in the
Erythrocebus patas, and Old World monkey. Amer. J. Pathol. 98:401-424,
1980.
Mattison,
D.R. and King, J.C.: Development of a nonhuman primate model for fetoscopy.
J. Med. Primatol. 12:319-330, 1983.
Page,
S.L., Chiu, C. and Goodman, M.: Molecular phylogeny of Old World monkeys
(Cercopithecidae) as inferred from gamma-globulin DNA sequences. Molec.
Phylogenet. Evol. 13:348-359, 1999.
Palmer,
A.E., London, W.T., Sly, D.L. and Rice, J.M.: Spontaneous preeclamptic
toxemia of pregnancy in the patas monkey (Erythrocebus patas).
Lab. Anim. Sci. 29:102-106, 1979.
Palmer,
A.E., London, W.T., Sly, D.L. and Rice, J.M.: Toxemia of pregnancy (preeclampsia,
eclampsia, hypertensive disorder of pregnancy). Chapter 88, pp. 213-215,
in, Spontaneous Animal Models of Human Disease. Vol. 1. E.J. Andrews,
B.C. Ward and N.H. Altman, eds. Academic Press, NY 1979.
Paterson,
M.: Biochemical parameters in the patas monkey. Lab. Anim. 9:21-31, 1975.
Panigel,
M., Brun, J.-L. and Pascaud, M.: Étude angiographique de la circulation
utero-placentaire chez les singes Cynomolgus (Macaca) irus et Erythrocebus
patas. Bull. l'Assoc. Anat., 52nd Cong. Paris-Orsay, pp. 965-957,
1967.
Panigel,
M. and Brun, J.-L.: Anatomie vasculaire, histologie et ultrastructure
du placenta a la fin de la gestation chez certains primates: Macaca
(Cynomolgus) irus et Cercopithecus (Erythrocebus)
patas. Bull. l'Assoc. Anatom. 53rd Cong., Tours, # 142, pp.1270-1286,
1968.
Panigel,
M.: Structure et ultrastructure compares de la membrane placentaire chez
certains primates non humains (Galago demidovii, Erythrocebus patas,
Macaca irus (fascicularis), Macaca mulatta et Papio cynocephalus).
Bull. l'Assoc. Anatom. 54thCong., Sofia # 145, pp.320-337, 1969.
Ramsey,
E.M., Corner, G.W. and Donner, M.W.: Serial and cineradiographic visualization
of maternal circulation in the primate (hemochorial) placenta. Amer. J.
Obstetr. Gynecol. 86:213-225, 1963.
Rice,
J.M., Williams, G.M., Palmer, A.E., London, W.T. and Sly, D.L.: Pathology
of gestational choriocarcinoma induced in patas monkeys by ethylnitrosourea
given during pregnancy. Placenta Suppl. 3:223-230, 1981.
Scott,
G.B.D.: Comparative Primate Pathology. Oxford University Press, Oxford,
1992.
Sly,
D.L., London, W.T., Palmer, A.E. and Rice, J.M.: Disseminated cryptococcosis
in a patas monkey (Erythrocebus patas). Lab. Anim. Sci. 27:694-699,
1977.
Sly,
D.L., London, W.T., Palmer, A.E. and Rice, J.M.: Growth and Hematologic
development of the patas monkey (Erythrocebus patas) to one year
of age. J. Med. Primatol. 7:156-164, 1978.
Sly,
D.L., Harbaugh, S.W., London, W.T. and Rice, J.M.: Reproductive performance
of a laboratory breeding colony of patas monkeys (). Amer. J. Primatol.
4:23-32, 1983.
Sulaiman,
S., Williams, J.F. and Wu, D.: Natural infections of vervet monkeys (Cercopithecus
aethiops) and African red monkeys (Erythrocebus patas) in Sudan
with taeniid cysticerci. J. Wildl. Dis. 22:586-587, 1986.
Wasmoen,
T. L., Benirschke, K. and Gleich, G.J.: Demonstration of immunoreactive
eosinophil granule major basic protein in the plasma and placentae of
non-human primates. Placenta 8:283-292,1987.
Wilson,
R.B., Holscher, M.A., Chang, T. and Hodges, J.R.: Fatal herpesviruses
simiae (N virus) infection in a patas monkey (Erythrocebus patas).
J. Vet. Diagn. Invest. 2:242-244, 1990.
Winterer,
J., Palmer, A.E., Cicmanec, J., Davies, E., Harbaugh, S. and Loriaux,
D.L.: Endocrine profile of pregnancy in the patas monkey (Erythrocebus
patas). Endocrinology 116:1090-1093, 1985.
Wolf,
R.H.: Placenta previa in a patas monkey (Erythrocebus patas). Folia
Primatol. 14:80-83, 1973.
|