| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: July 26, 2007. |
White-bellied Tree Pangolin
Manis tricuspis
Order: Pholidota
Family: Manidae
1) General Zoological Data
The classification of pangolins (appropriately the German name for pangolins is ‘Tannenzapfentiere’) as Pholidota is a recent event (Nowak, 1999). In the past they were contained under Edentata, with armadillos, sloths, anteaters and they have also been listed as genus Phataginus. They are now separated, and a closer relation to Carnivora has been suggested; more recent studies detailed by O’Brien et al. (2006), however, suggest otherwise. Seven distinct species are recognized, four Asian and three African pangolins. The name ‘Pangolin’ is a Malayan word and refers to ‘a roller’ because of their common feature to roll into a tight ball when disturbed or during the night. Manis comes from the Latin: manes (ghost) because of their nocturnal habit and their unusual appearance, and tricuspis is Latin for ‘three points’ (the ends of the scales in younger animals that wear down with age) (Gotch, 1979). The species shown here, Manis tricuspis has also been called Manis tridentata and it originates from West Africa. Numerous web sites with the distribution of this species and other details on all pangolins are available. In addition, an extensive review of pangolins in captivity has recently been provided by Yang et al. (2007). It contains much information on behavior and nutrition of captive animals.
The animals available to me came from Cameroon. This tree pangolin is the smallest pangolin species; it is generally a nocturnal and tree-dwelling animal with prehensile tail and its numbers have been decreasing in recent years, in part because of the bush meat trade. They are said to be very good in taste. Weigl (2005) reported that one animal had lived for 4 years and 2 months at the Arnhem Zoo (it arrived as adult), but animals are uncommonly held in zoos as their nutrition places significant demands on the zoo staffs (Puschmann, 2004). Only one set of twins has ever been reported and that was of the giant pangolin, but reproduction in captivity has been rare for all pangolin species. Pocock (1924) described the means by which one may differentiate the various pangolin species from the scale pattern of the under-side of the tail.
2) General Gestational Data
Single births are the rule in Manis tricuspis. The length of gestation is about 139 days according to Nowak (1999) and the newborn weighs around 100 g; thus, the gestation of the immature animals described here was about mid-way or slightly more. The sparse data collected so far have been categorized by Hayssen et al. (1993). When possible, these references have been searched but many are not accessible. The normal neonatal weight given in these papers is 90-150 g, and one born at Ife University Zoo (Nigeria) was 100 g (Menzies, 1967). The activities and feeding needs are there described as well. At 7½ months the young weighed 750 g. For nutritional details of white-bellied pangolins see Menzies (1963, 1966). These papers also refer to a brief report on such a birth at Cologne Zoo in 1962, but further details are unknown. The placental weight is unavailable for white-bellied pangolins and it was not determined in our first case as the entire uterus was fixed in formalin. In the second specimen, an abortus, the placenta weighed (4.5 g); the male fetus was 21.9 g with a crown-rump length of 7 cm. The progress and feeding were described in some detail in the reports to which I have referred.
Ogilvie & Bridgwater (1967) described the birth of an Indian pangolin (Manis crassicaudata) at the Oklahoma Zoo. They also referred to useful means of feeding their new arrivals and reported on the other births so far described. The birth of a Chinese pangolin (Manis pentadactyla) was reported by Masui (1967). Its placenta was expelled and was not consumed. It measured 24 x 3-6.5 cm, weighed 13 g and had a ‘multiplex’ appearance.
3) Implantation
Mossman (1987) described an antimesometrial implantation but early stages have not been described in detail. He also considered the placenta to be epitheliochorial, making reference to de Lange (see below).
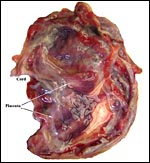 |
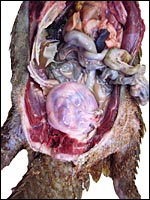
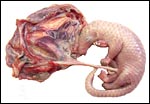
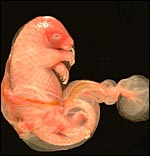
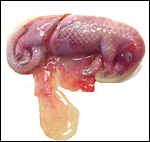
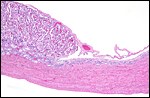
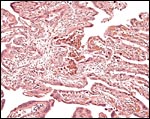
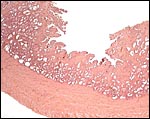
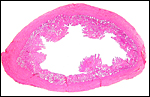
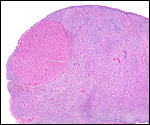
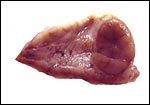
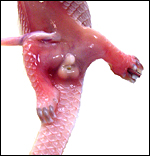
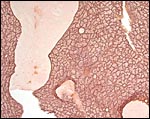
The placenta of our first case is still attached to the uterus. |
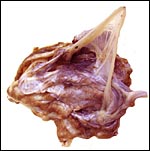 |
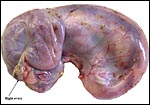
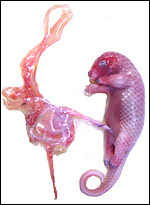
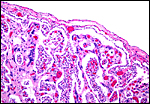
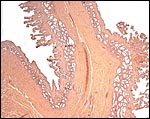
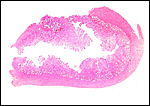
The amnion and other membranes are lifted off the uterus. |
4) General Characterization of the Placenta
Mossman (1987) described the placenta as being cotyledonary with an epitheliochorial relationship and with an antimesometrial first attachment. As will be seen from the photographs, the placentas of our gestations were ‘striped’, a description that was first introduced by Heath & Amachree (1967). This will be described in more detail subsequently. The pregnant uterus weighed 69.4 g; the male fetus weighed 44.5 g, had a 14 cm crown-rump and 10 cm tail length. The pregnant mother weighed 2,250 g. Unlike most other species I have examined, the endocervical tissue was unusually soft and a viscid yellow mucus exuded from the cut surface; it was also infiltrated on its superior aspects with trophoblast.
Heath & Amachree (1967) examined the placenta of the pangolin Manis tetradactyla in considerable detail, a Nigerian species that may by now have become extinct. They found differing structures than those often quoted and that originated with de Lange’s report (1931). In their specimen, the placenta was still contained within the uterus when the 2.25 kg animal died following leg amputation. The fetus was 18 cm long with 7 cm CR length and the placenta was arranged in 2-3 mm wide bands (‘strips’), much as we found in our specimens. Amniotic and allantoic fluids were clear and the placenta was located in the left uterine horn but extended for a short distance into the right horn. It had a “deep labyrinth and an underlying layer of distended endometrial glands. A narrow junctional zone was present containing syncytiotrophoblast. Throughout the labyrinth cytotrophoblast and syncytiotrophoblast were observed in contact with maternal capillaries. The placenta was considered to be endotheliochorial in type” and resembled that of carnivores. In an addendum, the authors detailed observations made on an older specimen that had essentially the same characteristics. Importantly, the superficial endometrium was invaded by trophoblast while the glands below remained intact and were secretory. These authors also disputed the ‘maternal’ origin of what de Lange had considered to be syncytial maternal epithelial cells and believed it to be trophoblastic in nature.
In my studies, this interpretation seems to be the correct one, rather than this being an epitheliochorial placentation.
5) Details of fetal/maternal barrier
De Lange (1931) described the placenta of Manis as being epithelio-chorial and likened it to that of the horse. Initially, a large yolk sac apparently develops with a large exocoelom, but they are being reduced by the growth of the allantois. The uterine epithelium, he described, is unremarkable but at the mouths of endometrial glands a proliferation of capillaries takes place and the uterine epithelium changes to become syncytial (so he described). The yolk sac and exocoelom are said to be retained to term and the villous tissue becomes arranged in longitudinal bands (‘strips’). De Lange goes into great detail regarding the maternal “villi” that precede the development of the fetal villi, that they are not entertwined and that the maternal component becomes superficially syncytial. In early stages there developed two uterine ‘cushions’ to which placenta tissue attaches.
My own observations suggest that the superficial endometrium is invaded by trophoblast and that delicate extensions carry maternal blood in between the fetal villi. There is no question but that there is a direct attachment of trophoblast to the delicate maternal connective tissue remaining from the endometrium and that, in most places, the trophoblast attaches to the endothelium of the maternal tissue extensions. This is true for both syncytiotrophoblast as well as, closer to the fetal surface, cytotrophoblast. There is no blood in the intervillous tissues, thus this is not a hemochorial organ. No pigment was found either. The cellular trophoblast also covers the underside of the chorion between the individual strips of villous tissue.
6) Umbilical cord
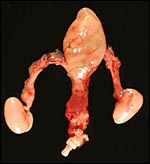
The umbilical cord of the first immature fetus was 4 cm long and had neither twists nor surface plaques. The amnion was directly around the fetus and in intimate contact. The second abortus’ umbilical cord measured 5.3 cm. The cord contains two large arteries, one vein and an allantoic duct that is lined by a single layer of urothelium. In addition, numerous small vessels are distributed throughout the cord, much as seen in dolphin, Artiodactyla, etc. The surface is made of a single layer of amnionic epithelium.
7) Uteroplacental circulation
The fetal vasculature is typical of the arrangement in endotheliochorial organs. No studies of the maternal circulation have been made.
8) Extraplacental membranes
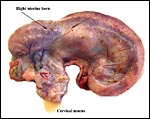
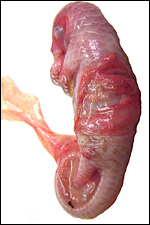
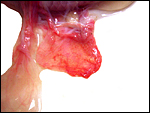
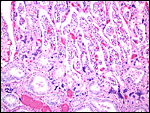
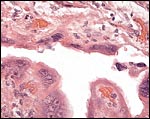
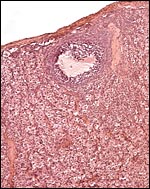
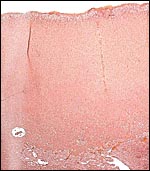
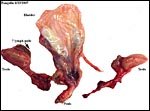
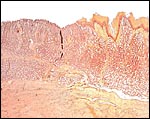
There is no decidua capsularis and the membranes are very thin and delicate. In the membranes shown next, the amnion is at the left and followed by chorion that contains large fetal vessels. Allantoic membranes follow next to the right and have only minimal vascularization.
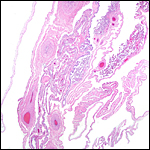 |
Membranes with amnion at left and large fetal vessels in chorion. |
9) Trophoblast external to barrier
There is no extravillous trophoblast, but at the base of the implanting placenta are typical syncytiotrophoblastic cells, also referred to as junctional zone. These had been interpreted by de Lange as being of endometrial epithelial origin, but that appears to be incorrect.
10) Endometrium
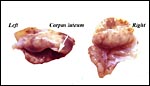
These species have a bicornuate uterus and the fetuses were contained within the left uterine horn, but the placenta extended for a short distance into the right horn and superior endocervix. There is no decidua. The corpus luteum was also on the left.
11) Various features
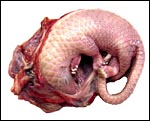
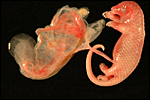
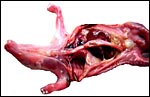
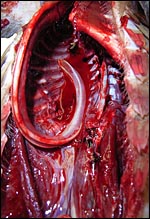
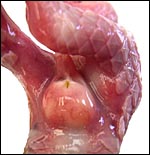
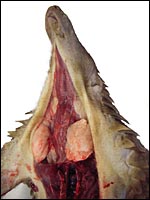
There is neither a ‘subplacenta’ nor is there invasive trophoblast. In fact, the attachment sites have distended endometrial glands beneath. The most unusual feature of pangolin anatomy is their tongue. It is accompanied by two cartilaginous sheaths and is very muscular. Moreover, it lies under the skin of the chest and enters the abdomen, where it has a marked terminal curvature. We did not find it attached to the pelvis as was described by earlier authors. These features are displayed in the next few photographs.
12) Endocrinology
In two of the adult animals available to us for autopsy, the testes had retracted into the abdomen and they have been described as being ‘inguinal’ in the literature; in this fetus, however, the testes had descended into the scrotum as is seen on the next picture. Mossman & Duke (1973) described some of the ovaries of pangolins. There is no bursa, the rete is large; there is an abundant thecal gland with much ovarian interstitial tissue. The adrenal gland is also large. The large pancreas found in white-bellied pangolins and the Malayan animal contained few islets of Langerhans only.
13) Genetics
O’Brien et al. (2006) were able to depict only the karyotypes of Manis javanica, with 38 chromosomes (mostly biarmed autosomes, a submetacentric X and a very small Y). They also referred to studies of M. pentadactyla from Asia that has been shown to possess 2n=36, 38, 40, 42, originating presumably from different regions in Asia and possibly related to one another by chromosomal fusions. Manis tricuspis has not yet been karyotyped to my knowledge but the specimens under discussion are currently being studied. Cell strains will then become available from CRES by contacting Dr. Oliver Ryder (oryder@ucsd.edu).
14) Immunology
I have not been able to find any published data.
15) Pathological features
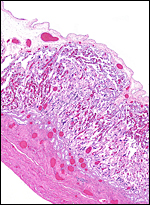
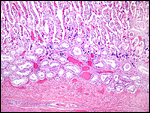
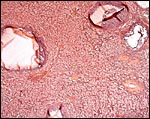
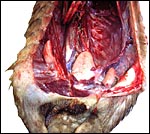
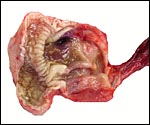
These animals from the San Diego Zoo came with tick-infestation and the dam of one animal had hepatic abscesses as well. In addition, long, thin round worms were present attached to an ulceration of the proximal stomach. Griner (1983) reported briefly on 4 autopsied animals, one of which was pregnant and died in 1967. The species denomination is not certain but they were most likely of Malayan origin. The cause of death in that animal was unclear and it was also undetermined for another animal studied by Griner. One animal had pancreatic necrosis and stomach impaction, and one had gastric ulcers and chronic interstitial nephritis.
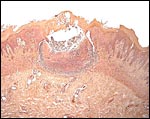 |
One of several vaginal ulcers in a Malayan pangolin. |
16) Physiologic data
As mentioned earlier, one of the most remarkable features are the length and attachment of the tongue, as these are ant- and termite-eaters. The slender tongue can measure up to 25 cm (Nowak, 1999) and it passes along the chest into the abdomen where it curves at its distal end (see picture of the dissected fetus and older animals above, as well as the description by Chan (1995). The muscular tongue resides in two flexible cartilaginous sheaths and can be propelled and withdrawn very rapidly. In addition, unusually large mucus-producing glands found in the mouth provide the mucus for the sticky tongue surface for the purpose of ant-eating. A detailed report on the feeding habits (Hymenoptera – ants; Isoptera – termites) of another African pangolin (Manis temminckii) comes from Swart et al. (1999). In addition, the review by Yang et al. (2007) gives much information on dietary requirements.
Another unusual feature of this edentulous animal is the squamous modification of the gastric mucosa that allows cracking of the ants and termites at the pylorus with a saw-like mucosal/muscular apparatus. Pangolins have a diminutive lower jaw and no zygomatic arches. These animals are also good swimmers and may fill their stomachs with air for buoyancy. In sections of the Malayan pangolin’s pancreas from Griner’s material and our adult white-bellied specimens I found only very sparse islets of Langerhans. The oral mucus glands had some distended ducts, and vaginal ulcers were present.
17) Other resources
Cell lines will become available of this animal in due time (see item # 13).
18) Other remarks – What additional Information is needed?
Early implantational stages and a better delineation of the arrangement of the placental ‘strips’ are needed.
Acknowledgement
The animal photographs of the Malayan pangolin come from the Zoological Society of San Diego.
References
Chan, L.-K.: Extrinsic lingual musculature of two pangolins (Pholidota:Manidae). J.
Mammal. 76:472-480, 1995.
De Lange, D. Jr.: Die Placenta der Edentaten. Verh. Anat. Ges. (Anat. Anz.) 71: Suppl. 234-236, 1931. (This is only a brief report at a meeting. The more extensive paper: Le placenta des Édentés. C.R. Ass. Anat. 25:189-199, 1930 was not accessible to me.)
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell’s Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Heath, E. and Amachree, M.: Endotheliochorial placentation in a Pangolin, Manis tetradactyla. Acta Zool. 48:309-315, 1967.
Masui, M.: Birth of a Chinese pangolin Manis pentadactyla at Ueno Zoo, Tokyo. International Zoo Yearbook 7:114-116, 1967.
Menzies, J.I.: A preliminary note on the birth and development of a Small-scaled tree pangolin Manis tricuspis at Ife University Zoo. International Zoo Yearbook 7:114, 1967.
Menzies, J.I.: Feeding pangolins, Manis spp., in captivity. International Zoo Yearbook 4:126-128, 1963.
Menzies, J.I.: A note on the nutrition of the tree pangolin, Manis tricuspis, in captivity. International Zoo Yearbook 6:71, 1966.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nowak, R.M.: Walker’s Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
O’Brien, S.J., Menninger, J.C. and Nash, W.G., eds.: Atlas of Mammalian Chromosomes. Wiley-Liss; A. John Wiley & Sons, Inc. Hoboken, N.J., 2006.
Ogilvie, P.W. and Bridgwater, D.D.: Notes on the breeding of an Indian pangolin Manis crassicaudata at Oklahoma Zoo. International Zoo Yearbook 7:116-118, 1967.
Pagès, É.: Notes sur la reproduction au Gabon de Manis tricuspis. Cycles Génitaux Saisonniers de Mammifères Sauvages. 151-164, 1968. Canivenc, R. ed. Masson & Cie, Paris. (I have not been able to access this article in a textbook.)
Pagès, É.: Comportement maternel et développement du jeune chez un Pangolin arboriocole (Manis tricuspis) Biol. Gabon 8:63-120, 1972. (I have not been able to access this interesting paper).
Pocock, R.I.: The external characteristics of the pangolins (Manidae). Proc. Zool. Soc. London, pp. 707-723, 1924.
Puschmann, W.: Zootierhaltung. Tiere in menschlicher Obhut. Säugetiere. Verlag Harri Deutsch, Frankfurt, 2004.
Swart, J.M., Richardson, P.R.K. and Ferguson, J.W.H.: Ecological factors affecting the feeding behaviour of pangolins (Manis temminckii). J. Zool. London 247:281-292, 1999.
Weigl, R.: Longevity of Mammals in Captivity; From the Living Collections of the World. [Kleine Senckenberg-Reihe 48]. E. Schweizerbart’sche Verlagsbuchhandlung (Nägele und Obermiller), Stuttgart, 2005.
Yang, C.W., Chen, S., Chang, C-Y., Lin, M.F., Block, E., Lorentsen, R., Chin, J.S.C. and Dierenfeld, E.S.: History and dietary husbandry of pangolins in captivity. Zoo Biol. 26:223-230, 2007.