|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
| posted: |
| July 8, 2002 |
Ailurus fulgens
Order: Carnivora
Family: Procyonidae (Ailurinae)
1) General Zoological Data
The lesser panda is currently recognized as an animal that best fits into a family of raccoon-like animals, the Procyonidae. It is a remarkably beautiful carnivore of central and East Asia. At least two subspecies are recognized, the commonly seen lesser panda Ailurus fulgens fulgens, and Styan's panda, Ailurus f. stayani, which is a slightly larger animal from the Eastern portion of the distribution. The animals inhabit mountainous woods in the Himalayans and prefer colder temperature (Nowak, 1999). Adults weigh from 3-6 kg and may live to more than 17 years in captivity. Although belonging to the Order Carnivora, most food consumed by lesser pandas is vegetarian, with eggs and small rodents occasionally also taken. Females are said to be in estrus only once a year, for one day. A comprehensive review of contributions to the biology of the red panda was edited by Glatston (1989).
The lesser pandas are endangered species (CITES I category) with as few as 2,500 animals estimated to survive. They are frequently seen in zoos and make attractive exhibits. Another complete review of this species can thus be found by Pagel (1996) from the Cologne zoo in Germany. It includes information on the animal's distribution in the wild, its reproduction in the Cologne Zoo, behavior and feeding practices. Another, more popular, review is that by Roberts (1982) in which the 4.5 Million year history of the species is discussed and the origin of its name (shining cat) mentioned.
The old reference to "cat" exemplifies the taxonomic problems that have existed with the two panda species. Mayr (1986) discussed this controversy in lucid and at times funny detail that is eminently readable. He concluded, more or less, that the putative relation that was thought to exist between giant and lesser panda is the result of both animals coming from China and also, because both are called "panda". In Germany, where the giant panda is referred to as Bambusbär (bamboo bear), this idea has not surfaced. Most of the literature concerning the evolution of these two species of panda can be found referred to in the commentary by Mayr (1986). But these ideas may have been oversimplified as, since then, additional evidence has been gathered and the data from which these conclusions had been drawn have been reinterpreted. Moreover, many new studies have been undertaken. For instance, in a searching study of bile acid substitutions, Hagey et al. (1993) showed that all bears have ursodeoxycholic acid, but not the giant panda nor, of course, the lesser panda. The bile acid composition of the lesser panda is much closer to that of a raccoon but, here again, the giant panda differs. Chromosomal banding patterns of various carnivores by Wurster-Hill & Gray (1975) had shown that the procyonids, including the lesser panda, had patterns that most simulated those of felidae; these authors also discussed the putative ancient origins of these species. Tagle et al. (1986) constructed a molecular phylogeny of carnivores from protein sequences. These separate the giant panda widely from ursidae and relate them more closely to the lesser panda and closer to the procyonidae. Zhang & Ryder (1993) studied mtDNA sequences of pandas and other Carnivora and came to this conclusion "…the giant panda is more closely related to bears than to the lesser panda; the lesser panda is neither closely related to bears nor to the New World procyonidae". Flynn et al. (2000) have incorporated a large variety of Carnivora in their searching inquiry and they concluded that the lesser panda is neither closely related to the bear, nor to the procyonids but that it has more affinities with mustelids (see also Flynn & Nedbal, 1998, and Slattery & O'Brien, 1995). Nie et al. (2002) came to the same conclusion by studying chromosome painting of various possibly related species. Chromosomal painting studies by Tian et al. (2002) suggested that the karyotype has much homology to that of the cat. It is thus probably safe to say that the last word has not been written on the ultimate classification of these two species. The placental findings that are related here indicate that there are great similarities to the placentas of mustelids.
 |
Red panda at San Diego Zoo. |
 |
Another lesser panda at San Diego Zoo. |
 |
Another red panda at San Diego Zoo. Note the characteristic long, ringed tail. |
The reproductive biology of lesser pandas has been described in admirable detail by Roberts & Kessler (1979) but many details still need to be filled in. The animals apparently have a clear seasonality but there is indication also that the reproductive proclivity may vary with latitude and perhaps with altitude. From the only, albeit small data of steroid secretion by Spanner et al. (1992) it appears that the red panda is most likely a reflex ovulator. The length of gestation is 114-145 days (but once recorded to be only 90 days). The possibility that delayed implantation and/or possible mating during pregnancy are two factors that have been considered to explain the wide span of gestational dates (Puschmann, 1989; Nowak, 1999). One to two young are born most commonly; very rarely are there more offspring. Newborns weigh around 200 g. The uterus is bicornuate and ovaries are enclosed in a bursa (Mossman & Duke, 1973).
The male animal whose placenta is shown here weighed 180 g, and its stillborn sibling was 108 g. It was well preserved and the cause of its death is unknown.
 |
The stillborn twin of the gestation described here. |
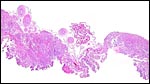
No detailed studies on ailurid implantation are known to me, and Mossman (1987) stated that no panda placenta of the lesser panda had been described. There are a number of descriptions of raccoon placentas, however. Their placenta is most closely of an endothelio-chorial character, although a "vaso-chorial relation has been suggested to be a better name. That is because there is a prominent basement membrane of the fetal sinusoids. The placenta of raccoons falls into the general arrangement of carnivore placentas (Amoroso, 1961).
The placenta of the red panda is also very different from the placenta of dog and cat (see those chapters). The filiform arrangement of villi, for instance, is lacking and the placenta does not have the ring-shaped, zonary form seen in many other carnivore species. It is rather discoid with a small central sulcus connecting the villous tissue with a strip of membranes and, in its histology, it also appears to me more similar to the placenta of the primitive carnivore Zorilla striata, described by Rau (1925). A nearly bidiscoid (rather than zonary) placenta was found in that species, and the lesser panda placenta is otherwise also not too much different in structure. The hypertrophy of maternal capillary endothelium and gross morphology are nearly identical to all the carnivore species placentas but they are quite different from those of cats. It must be emphasized, however, that it will be essential to witness an implanted placenta in order to be able to rule out a more ring-shaped organ than that here portrayed. In further consideration of this morphology it is interesting to note that some genetic studies have also likened the lesser panda to mustelids (see above).
It is noted that possible yolk sac vessels are seen in the cord but no remnant of yolk sac morphology is identified in the specimen studied by me.
4) General Characterization of the Placenta
As indicated earlier, I have been unable to find any record of a red panda placenta in the literature. This may thus be its first description. This placenta was detached from the surviving neonate very shortly after its birth and was thus well preserved. It is not known whether the mother consumed any of it, although the organ appeared to be complete. It weighed 5.9 g and measured 4.5 x 3.5 cm, with a 3.5 cm umbilical cord and torn membranes. It had a discoid shape, unlike than the completely zonary placenta of raccoons, dogs and cats. There also was no green discoloration at the edge, as is seen in dog and cat, a feature whose lack was already remarked upon for the raccoon placenta by Watson (1881). Biggers & Creed (1962) were critical of Watson's study and subsequently obtained much better material for study. They mention for instance, that although affirming the zonary nature of the placenta (being certain that it was not interrupted), they were certain that the "epitrichium" described by Watson was actually the closely-applied amnion. Moreover, they were the first to introduce the notion of the "haemophagous organ". It exists prominently in young gestations but gradually involutes towards term. Here, trophoblast is believed to invade the uterus, thus creating focally a hemochorial organ and depositing much pigment. In later studies (Creed & Biggers, 1963; 1964) the authors identified similar structures in several mustelidae and suggested that it is used for red cell phagocytosis of the fetus. These species also had completely annular placentas.
The delivered red panda placenta was 2-3 mm thick and the maternal surface was ragged without any recognizable maternal components. It is noteworthy that the raccoon placenta, as already described in Watson's remarkable paper (1881), was zonary - "The foetus was encircled by an annular or zonary placenta…". The same author, however, also mentions the existence of a "gap" in the placenta and that this "gap" is much more pronounced in mustelids. Thus, the discoid feature of the lesser panda placenta separates the structure from raccoons and certainly from cat and dog, and perhaps the gross morphology allies it more closely to that of mustelids. This is questionable only because some of these are described as being zonary as well and as having a haemophagous organ in young gestations of which there was no direct evidence in this term placenta.
In this delivered placenta, the surface vessels were very prominent. Some other vessels that attached to the membranes are not truly identified. They may be the remains of vitelline vessels, as existing in some mustelidae (e.g. the wolverine - see Wislockie & Amoroso, 1956) but I am not certain of this.
The lesser panda placenta was a delivered, detached organ, much like the detached zonary raccoon placenta described by Watson (1881). He then already remarked on the "deciduate" nature of the placenta; that is to say, a portion of maternal tissue is shed with detachment, as is the case in other carnivora. In our case, the maternal tissue most easily recognized as having been shed are the large number of maternal vascular tubes shown in more detail in the histologic description, the "labyrinth". As was true of Watson in the raccoon placenta, I was unable to identify other maternal (decidua-like or glandular) tissues in the detached placenta. It must be emphasized though that the histologic structure is of course very different from that of the artiodactyl placenta where the fetal placental villi are dislodged from their caruncular tissue recesses, and very little maternal tissue remains with the delivered placenta.
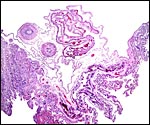
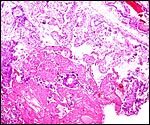
This is a labyrinthine organ with a somewhat confusing histology. Large maternal vascular channels of nearly uniform diameters traverse to the undersurface of the chorion, even in the delivered organ, as this is a "deciduate" placenta. These channels are generally referred to as "capillaries" but are much too large for that description, as was already stated by Amoroso (1961). They are lined by a much hypertrophied, cuboidal endothelium that is placed on a prominent basement membrane. This basement membrane, if analogous with that of the raccoon placenta, stains deeply with PAS (Creed & Harrison, 1965). Because of the prominence of this basement membrane the designation "vaso-chorial" placenta has been used, rather than "endothelio-chorial".
The trophoblast is only focally syncytial but is usually cuboidal and relatively thin. It covers the connective tissue of the very inapparent villous connective tissue. I did not encounter any binucleate trophoblastic cells. In this specimen, most of the maternal blood had been drained and, therefore, most blood seen in the placental sections was of fetal origin. There has long been a controversy regarding the nature of the syncytial cells - maternal or fetal? As stated in other chapters, the arguments have swung in favor of a trophoblastic derivation (see Amoroso, 1961).
Beneath the labyrinth one can expect to find the transitional or junctional zone. This is the region in which the placenta separates but little of this region is present in the detached organ. It contains degenerate material and lies above the much hypertrophied endometrial glands of the decidua basalis. The glands are generally obstructed by the invading placenta. It is speculated that some of the degenerated material of the junctional zone in carnivores is absorbed for fetal nutrition.
The umbilical cord measured 3.5 cm in length and was only slightly spiraled. (Watson (1881) remarked how short the cord in the raccoon was). It contained two arteries, a vein and a large allantoic duct, similar to that of raccoons. No omphalomesenteric duct was present. There were numerous small capillary vessels, as are seen in so many ungulate cords. In this placenta, however, they were strictly concentrated around the allantoic duct. Some of these vessels had a considerable musculature. The allantoic duct was also accompanied by many bundles of smooth muscle. Its lining was urothelial.
There have been no studies of the placental circulation in the lesser panda.
8) Extraplacental membranes
The amnion is covered, for the most part, with a thin squamous epithelium. Here and there, a few patches of squamous metaplasia are present. The amnion is avascular and is loosely attached to the allantoic sac. Its connective tissue contains ample vasculature and its epithelium is flattened, urothelium-like. No remnants of yolk sac structures were identified.
Inasmuch as no uterus has yet become available, one cannot make a judgment as to the depth of trophoblastic invasion into endo/myometrium. If, however, the analogy of placental structure to that of mustelids is correct, one would expect trophoblastic invasion of the endometrium and focal regions of destruction, but not of the myometrium.
10)
Endometrium
No uterus has been available and it is, therefore, unknown whether true
decidua is formed, whether it is invaded by trophoblast, or predict the
existence of any other unusual features. They will need to be gathered
from future material. In analogy with the mustelid placenta, however,
degenerated material (a former "hematoma"?) is found in the
presumptive "transition zone" (that comparable to dog and cat).
Watson (1881) who described the raccoon placenta, stated that a lush decidua
was present in the non-pregnant horn of the uterus he examined.
While these twins observed were of different sex and were thus surely dizygotic, the relationship between the two placentas is unknown. It is not even known whether the placentas were implanted in different uterine horns. The placenta of the stillborn fetus was apparently consumed by the mother and was not available. Much more reproductive anatomic study is needed.
12) Endocrinology
Only one published report of endocrine studies on red pandas is known to me (Spanner et al., 1997). This suggests that ovulation is followed by an estrogen peak and then followed by a rise in fecal progesterone derivative levels. The study suggested that red pandas may be reflex ovulators, i.e. ovulate only after mating.
13) Genetics
The lesser panda has 36 chromosomes (Todd & Pressman, 1968; Wurster-Hill & Gray, 1975). Because of the dwindling population and because of the availability of zoo records, Princée (1988) studied the relationships of captive lesser pandas. He suggested that there is a diminishing gene pool and that migration of specimens among zoos should take place. Su et al. (2001) on the other hand examined mtDNA haplotypes of 53 Chinese pandas and found considerable genetic diversity in the wild and published data that suggested to them that the population of wild Chinese lesser pandas is expanding southward.
 |
Karyotype of male panda. |
 |
Karyotype of female lesser panda. |
14)
Immunology
I am not aware of any definitive studies in this species.
15)
Pathological features
A number of diseases are known to afflict red pandas. The best review
of a variety of conditions is that by Montali et al. (1984) who reviewed
the causes of death of 52 animals at the National Zoo in Washington, DC.
Surprisingly, there were three congenital anomalies (anencephaly, truncus
arteriosus and hypomelia). Infectious diseases, mostly respiratory, were
the principal causes of death in this collection as well as reported from
other zoos. In addition, however, there were many deaths (25) of juveniles.
Other authors have remarked on high perinatal mortality rates and difficulties
in raising youngsters. Various parasitic afflictions and two tumors (mastocytoma
and leukemia) were described as well. It is also important to point to
the number of reports that show distemper mortality following vaccination.
Apparently pandas are quite sensitive to the modified virus. Their sensitivity
to heat and humidity were also stressed by the authors.
Various additional pathologic conditions have been reported. Thus, Langan
et al. (2000) described a nine-year old panda as having died from Tyzzer's
disease (intestinal infection with Clostridium piliforme). Kearns
et al. (1999) found skin lesions due to Microsporum gypseum in
many captive animals. They responded to antifungal therapy. A fatal abscess
reported by Dyer et al. (2000) grew only Chromobacterium violaceum,
a common soil organism. Poelma (1975) found infection with Pneumocystis
carinii in many animals, including red pandas, and Griner (1983) found
tuberculosis in a recently imported animal.
16) Physiologic data
I have not been able to find any significant physiological studies to
have been carried out in red pandas. Schweigert et al. (1990) examined
the transport of vitamin A in carnivores, including the lesser panda.
It was found that circulating vitamin A is bound to lipoproteins and reaches
levels in carnivores that, in other mammals would be toxic. The study
on bile acid composition by Hagey et al (1993) has been referred to at
the beginning of this chapter.
17) Other resources
Cell lines of many lesser pandas and of both subspecies are available
from CRES
at the Zoological Society of San Diego by contacting Dr. Oliver Ryder
at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
I quite expect that additional studies will be published to further define
whether the lesser panda is a procyonid or more of a mustelid. Perhaps
it deserves its own status in the classification of animals. Since this
is the first description of its placenta one can only hope that in the
future an implanted placenta will be described. Also, is there any evidence
of delayed implantation? Much more endocrine work is needed.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall's Physiology
of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown
& Co. Boston, 1961.
Biggers, J.D. and Creed, R.F.S.: Two morphological types of placenta in the raccoon. Nature 194:103-105, 1962.
Creed, R.F.S. and Biggers, J.D.: Development of the raccoon placenta. Amer. J. Anat. 113:417-445, 1963.
Creed, R.F.S. and Biggers, J.D.: Placental haemophagous organs in the procyonidae and mustelidae. J. Reprod. Fertil. 8:133-137, 1964.
Creed, R.F.S. and Harrison, R.J.: Preliminary observations on the ultrastructure of the raccoon (Procyon lotor) placenta. J. Anat. 99:933, 1965. {Abstract only}
Dyer, N.W., Krogh, D.F., DeVold, R., Wilson, S.L. and White, D.G.: Chromobacteriosis in a Chinese red panda (Ailurus fulgens styani). J. Vet. Diagn. Invest. 12:177-179, 2000.
Flynn, J.J. and Nedbal, M.A.: Phylogeny of the Carnivora (Mammalia): congruence vs. incompatibility among multiple data sets. Mol. Phylogenet. Evol. 9:414-426, 1998.
Flynn, J.J., Nedbal, M.A., Dragoo, J.W. and Honeycott, R.L.: Whence the panda? Mol. Phylogenet. Evol. 17:190-199, 2000.
Glatston, A.R., ed.: Red Panda Biology. SPB Academic Publishing, Amsterdam, 1989.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hagey, L.R., Crombie, D.L., Espinosa, E., Carey, M.C., Igimi, H. and Hofmann, A.F.: Ursodeoxycholic acid in the ursidae: biliary bile acids of bears, pandas, and related carnivores. J. Lipid Res. 34:1911-1917, 1993.
Kearns, K.S., Pollock, C.G. and Ramsay, E.C.: Dermatophytosis in red pandas (Ailurus fulgens fulgens): a review of 14 cases. J. Zoo Wildl. Med. 30:561-563, 1999.
Langan, J., Bemis, D., Harbo, S., Pollock, C. and Schumacher, J.: Tyzzer's disease in a red panda (Ailurus fulgens fulgens). J. Zoo Wildl. Med. 31:558-562, 2000.
Mayr, E.: Uncertainty in science: is the giant panda a bear or a raccoon? Nature 323:769-771, 1986.
Montali, R.J., Roberts, M., Freeman, R.A. and Bush, M.: Pathology survey of the red panda (Ailurus fulgens). Chapter 13 (pp. 128-140) in, "One Medicine", O.A. Ryder and M.L. Byrd, eds. Springer-Verlag, New York, 1984.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nie, W., Wang, J., O'Brien, P.C., Fu, B., Ferguson-Smith, M.A. and Yang, F.: The genome phylogeny of domestic cat, red panda and five mustelid species revealed by comparative chromosome painting and G-banding. Chromosome Res. 10:209-222, 2002.
Pagel, T.: Der kleine Panda (Ailurus fulgens) - Haltung und Zucht im Zoologischen Garten Köln. Z. des Kölner Zoo 39:139-155, 1996. (In German)
Poelma, F.G.: Pneumocystis carinii infections in zoo animals. Z. Parasitenk. 46:61-68, 1975.
Princée, F.P.G.: Genetic variation in the zoo population of the red panda subspecies Ailurus fulgens fulgens. Zoo Biol. 7:219-231, 1988.
Puschmann, W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag Berlin, 1989.
Rau, A.S.: Contributions to our knowledge of the structure of the placenta of mustelidae, ursidae, and sciuridae. Proc. Zool. Soc. London 25:1027-1070, 1925.
Reddacliff, G.L., Halnan, C.R.E. and Martin, I.C.A.: Mosaic 35,X/36,XY karyotype and intersex in a red panda (Ailurus fulgens). J. Wildl. Dis. 29:169-173, 1993.
Roberts, M.: The red panda: its history and fragile hold on the future. Your Cincinnati Zoo News Spring/Summer 1983, pp. 1-5.
Roberts, M. and Kessler, D.S.: Reproduction in red pandas, Ailurus fulgens (Carnivora: Ailuropodidae). J. Zool. Lond. 188:235-249, 1979.
Schweigert, F.J., Ryder, O.A., Rambeck, W.A. and Zucker, H.: The majority of vitamin A is transported as retinyl esters in the blood of most carnivores. Comp. Biochem. Physiol. 95A:573-578, 1990.
Slattery, J.P. and O'Brien, S.J.: Molecular phylogeny of the red panda (Ailurus fulgens). J. Hered. 86:413-422, 1995.
Spanner, A., Stone, G.M. and Schultz, D.: Excretion profiles of some reproductive steroids in the faeces of captive Nepalese red panda (Ailurus fulgens fulgens). Reprod. Fertil. 9:565-570, 1997.
Su, B., Fu, Y., Wang, Y., Jin, L. and Chakraborty, R.: Genetic diversity and population history of the red panda (Ailurus fulgens) as inferred from mitochondrial; DNA sequence variation. Mol. Biol. Evol. 18:1070-1076, 2001.
Tagle, D.A., Miyamoto, M.M., Goodman, M., Hofmann, O., Braunitzer, G., Göltenboth, R. and Jalanka, H.: Hemoglobin of pandas: Phylogenetic relationships of carnivores as ascertained with protein sequence data. Naturwissenschaften 73:512-514, 1986.
Tian, Y., Nie, W.H., Wang, J.H., Yang, Y.F. and Yang, F.T.: Comparative chromosome painting shows the red panda (Ailurus fulgens) has a highly conserved karyotype. Yi Chuan Xue Bao 29:124-127, 2002. (In Chinese)
Todd, N.B. and Pressman, S.R.: The karyotype of the lesser panda (Ailurus fulgens) and general remarks on the phylogeny and affinities of the panda. Carnivore Genet Newsl. 5:105, 1968.
Watson, M.: On the female organs and placentation of the raccoon (Procyon lotor) Proc. Roy. Soc. London 32:272-298, 1881.
Wislockie, G.B. and Amoroso, E.C.: The placenta of the wolverine (Gulo gulo luscus) (Linnaeus). Bull. Mus. Compar. Zool. (Harvard). 114:93-100, 1956.
Wurster-Hill, D.H. and Gray, C.W.: The interrelationships of chromosome banding patterns in procyonids, viverrids, and felids. Cytogenet. Cell Genet. 15:306-331, 1975.
Zhang, Ya-Ping and Ryder, O.A.: Mitochondrial DNA sequence evolution in the arctoidea. Proc. Natl. Acad. Sci. 90:9557-9561, 1993.