| (Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.) |
| Last updated: Feb. 27, 2004 |
Dinomys branickii
Order: Rodentia
Family: Dinomyidae
1) General Zoological Data
The pacarana is the third-largest rodent of South America and is the only member of the family Dinomyidae. It is primarily nocturnally active, generally lives in burrows but has otherwise a "porcupine-like behavior". It is especially prone to climb trees and likes to rest on elevated platforms when in captivity. Pacaranas are found primarily in Colombia, Peru, Bolivia and Ecuador, on the lower slopes of the Andes (Collins & Eisenberg, 1972). Meritt (1984) has reviewed most of what is known about the physiology and behavior of this hystricognath (caviomorph) rodent. He suggested that the name 'pacarana' is the Tupi word for 'false paca'. [In concordance with the recommendations by Wilson & Reeder, 1992, I will here refer to the caviomorph rodents as hystricognaths]. The fossil history of pacaranas is unknown but the animal appears to be a close relative of the agouti, as was hypothesized by Simpson (1980). The longevity in captivity is at least 13 years. Additional information may be found in the description by Nguyen. The ovaries of pacaranas display most unusual features. They are extremely lobulated but have not been described before.
Only few zoos have displayed this nocturnal animal and thus much more needs to be learned about its physiology. At present, a very actively reproductive colony of animals is being maintained at the San Diego Zoo; it originated with imports from Colombia. These animals have lived in the past with a Goeldi's monkey most harmoniously; they are also mostly active at night. They are easily trained to perform in shows and tend to sit upright to consume food that is grasped between their front paws.
 |
Two pacaranas at San Diego Zoo in their typical eating posture. |
 |
Two pacaranas eating sweet potatoes. |
 |
Juvenile pacarana at San Diego Zoo. |
Collins & Eisenberg (1972) described some details of the Cesarean section performed in a pacarana at the National Zoo in 1971. The dam weighed 16 kg and two dead fetuses were removed by this operation (one actually had a partially aerated lung at autopsy). The weights, with placenta attached, were 1,076.5 g and 835 g respectively. The section of one of these placentas was available to me and it is here also described. Twins and triplets are commonly born, but singletons and quadruplets occur as well. The exact length of gestation is still unknown. This is so despite our zoo having an excellent keeper who was very familiar with this group of animals. She never saw copulation and was thus unable to judge the length of gestation precisely. Others have estimated it to vary between 230 and 283 days, certainly representing a very long period. Meritt (1984) stated from own observations that the gestation is maximally 254 days long. He also recorded the weights of three neonates: 570, 605, 660 g. A male neonate that we were able to weigh was 602 g; two other singletons were 700 and 730 g. The latter weighed only 596 g one week later when it died.
In our zoo, the placenta was always eaten by the dam after giving birth and none had become available from our colony despite my many attempts to obtain a specimen. Therefore, no placental weights were available, except those of Collins & Eisenberg's study of stillborn fetuses that were weighed including the placenta. Additionally, however, a post partum uterus was available that displayed interesting features to be described. Since pasting these initial pages two full-term gestations were observed. One was a term stillborn whose placenta was still attached; another was delivered by Cesarean section.
3) Implantation
Early stages of pregnancy have not been observed. In fact, virtually nothing is known about pacarana gestations. Reasoning by analogy from the several other hystricognaths studied, it is likely that this animal also has a mesometrial implantation and a generally similar placentation to that of other hystricognaths (Luckett, 1980). For that reason, I show the immature gestation of a chinchilla uterus below that explains clearly the manner of membrane formation. It does not have as lobular a disk as that present in pacarana, however.
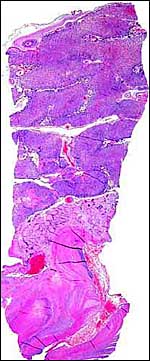
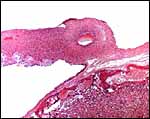
The pacarana has a bicornuate uterus and both horns are used for the gestation. The data and sections of an immature fetus and placenta shown here were kindly made available by Dr. Hiroaki Soma, of Tokyo, Japan. The slides of this specimen were obtained from the Izu Cactus Garden where a thriving colony of pacaranas once existed. This abortus was delivered in 1990. It is obviously a very immature specimen. The mature specimen discussed here comes from the National Zoo and represents the placenta of a male that was delivered by Cesarean section (see above). These slides were kindly made available by Dr. R. Montali.
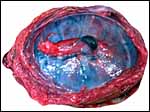
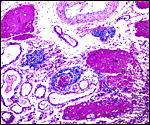
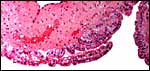
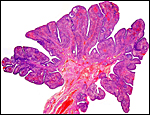
As stated, although early stages have not been available, it seems likely that they would be very similar to those described for other hystricognath rodents. The reader is therefore referred to my chapter on the Canadian porcupine and to the reviews by Nanaev et al. (1995), Luckett (1980), and Luckett & Mossman (1981). The placenta of the pacarana is a discoid, labyrinthine, hemo-monochorial organ with inverted yolk sac and with a large subplacenta. It has a discoid shape and is depicted below.
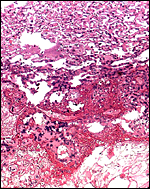
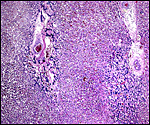
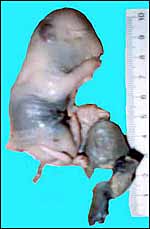 |
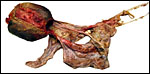
Aborted specimen of immature pacarana still attached to its placenta. Specimen from Izu Cactus Garden, Japan. |
No placenta of the pacarana has been described previously. To fill in the gaps of our knowledge, it is imperative that more mature placentas be obtained for additional study and that implantational stages also become available. In many respects, the immature placenta shown here has many similarities to the porcupine placenta (see that chapter), except for the absence of a typical subplacenta, at least that was the case in this immature placenta from Japan. That is not to say that there exists no subplacenta in immature placentas of the pacarana, as this is otherwise so typical of the hystricognath rodents; indeed, the mature specimens from the National Zoo shown subsequently as well as our new specimens, show this subplacenta well. This feature badly needs additional study to better understand its evolution.
The lobular nature of the placenta is very similar to that of some other members of this group but it differs considerably from the structure of the guinea pig placenta. The similarity is perhaps greatest when compared to the placental structures described in capybara, agouti and paca (Miglino et al., 2002). Those placentas are also strikingly lobulated and have "interlobia" that are composed of trophoblastic giant cells that radiate towards the centers of the lobules. Likewise, fetal connective tissue and arteries are found in the interlobium. These species also possess subplacentas. Luckett & Mossman (1981) suggested that the larger the fetus is in rodents, the more lobulated the placenta becomes. Perhaps that is the reason for the markedly lobulated organ in the pacarana.
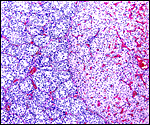
The subplacenta is remarkable. It is composed of a broad zone that was detached from the uterus at delivery in the specimen available. It contains a very large number of huge multinucleated giant cells and much amorphous material whose origin is not apparent. This region lies directly below the lobular disk and is traversed by maternal vessels, some of which are infiltrated by extravillous trophoblast. In contrast to the material from the porcupine, no pigment is present. Authors have speculated that this region may be responsible for the production of steroid hormones but I see no conclusive evidence for this. The appearance of these cells is not like that of endocrine cells. Others attributed possible local gonadotropin production to the subplacenta, while some investigators have attributed nutrient effects to this region. It has even been considered that these cells are of granulomatous nature, but that is clearly not the case. Luckett (1977) suggested that the subplacenta may represent a growth zone for the placental disk. The vastly different structure of these regions, however, suggests otherwise. But the real function of this subplacental tissue, and the nature of the amorphous material remain to be elucidated. Other possibilities are considered in the discussion by Luckett & Mossman (1981). Kaufmann (pers. comm.) suggested that this region represents an important zone from placental/trophoblastic growth.
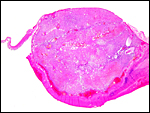
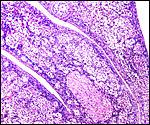
Since this preliminary description of the pacarana placenta two superb specimens have become available. Their features are shown in the next slides. This dam was once found to have delivered a stillborn fetus at term and next, she was found to have discharged blood one morning and a radiograph disclosed a term gestation. A Cesarean section was performed and a fresh placenta from a singleton in the right uterine horn was obtained. The neonate survived for only one week and then died with pneumonia. This placenta weighed 76 g, measured 7x6x2 cm and had a 3cm umbilical cord attached. The maternal surface was quite smooth and only in its center was there some disruption from the "stalk" leading to the subplacenta. Similarly, in serial sections, this central region of the placenta with its numerous giant cells and debris was firmer and white. The stillborn fetus of an earlier gestation will be shown next. It weighed 700 g and the placenta was 73 g with the tattered membranes attached. Reasons for the stillbirth could not be ascertained.
The pacarana placenta, like that of other hystricognaths (Roberts & Perry, 1974), has a hemomonochorial type of placentation where, for the most part, the two blood streams are separated by a layer of syncytial trophoblast only. One of the striking features of the pacarana placenta is the presence of the complex "subplacenta". Furthermore, the vascular invasion of trophoblast, deep into the myometrium, is a remarkable aspect that has found extensive attention primarily in the guinea pig by several investigators (summarized in detail by Nanaev et al., 1995). The drawing of the porcupine placentation (see the chapter on porcupine) applies largely to the pacarana placenta as well.
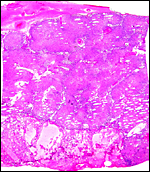
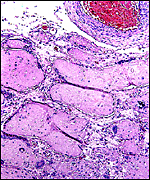
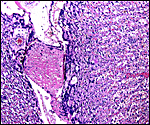
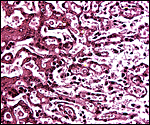 |
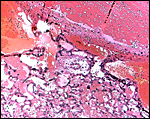
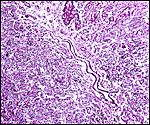
Labyrinth of immature pacarana placenta. |
The length of the umbilical cord was previously unknown. In the small fetus depicted above it is very short and its amnionic surface did not exhibit areas of squamous metaplasia histologically, as is so often found in many other species. None were also found in the porcupine (see that chapter), but unlike that species, small blood vessels were absent in the pacarana umbilical cord. There also were no capillaries around the allantoic duct.
The new placentas of the term gestation had a 3 cm umbilical cord inserted in its center. They had no spirals and there were no areas of squamous metaplasia. In these term cords there was no allantoic duct but there was a very large number of amuscular small blood vessels.
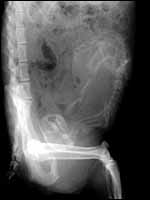
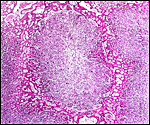
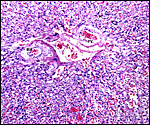
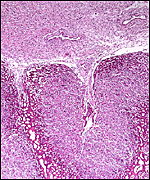
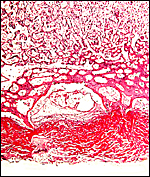
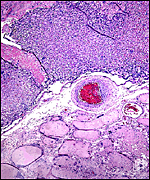
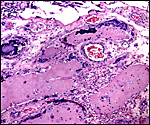
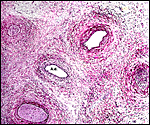
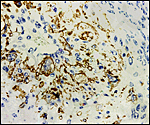
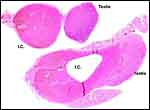
The arterial supply to the placenta is remarkable. There was a small amount of decidua attached to this immature placenta, and that showed arterial invasion by trophoblast. More interesting are the findings of a post partum uterus I examined together with Dr. Dale Agnew at the San Diego Zoo. The dam had 15 previous gestations and was euthanized after hysterectomy undertaken following delivery of her 600 g offspring that died soon after birth. She had severe renal disease from ureteral fibrosis. The uterine arteries that were deep in the myometrium were virtually completely obstructed by large masses of trophoblast. The vessels were surrounded by a lympho-plasmacellular infiltrate. The endometrium had regenerated (one month after delivery) and contained much hemosiderin.
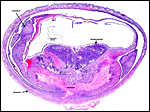
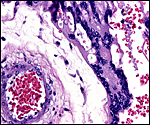
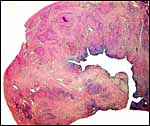 |
Post partum uterus with deep infiltration of trophoblast into maternal myometrial blood vessels. |
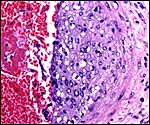
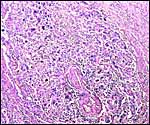 |
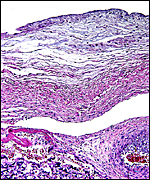
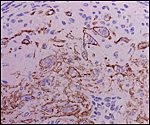
Myometrial artery surrounded by persisting trophoblast in post partum uterus described above. |
There was too much autolysis present in the immature specimen to make detailed observations. The outer surface has a columnar vitelline epithelium of the inverted yolk sac. I presume that this lies against the maternal endometrium. The mature specimen had no membranes for study. More detailed study can only come from an implanted placenta where the membrane attachment to the uterus can be studied. The amnion is likely to develop by cavitation, as is true in all "caviomorphs" (Luckett, 1980). It is avascular and has a flat epithelium.
As has been indicated earlier, and as is true of other hystricognath rodents, this uterus is infiltrated by large numbers of trophoblastic cells that are especially deeply invasive into and around the myometrial arterioles. They are cytokeratin-positive and very pleomorphic.
I have not been able to identify true decidualization in these specimens because of their detachment from the uterus. In the hystricomorph placentas, however, decidualization takes place that differs greatly from what is known in human gestations. It borders the junctional giant cell zone of the subplacenta with its complex structure.
11)
Various features
Hystricognath rodents have a pronounced subplacenta that is attached to
the endometrium. While the sections of the immature placenta did not have
a subplacenta, it was well developed in the Cesarean-sectioned mature
specimen, however. Additional studies are needed to clarify the development
of the subplacenta in pacaranas and this requires attached organs and
several stages of gestation. There is no reason to believe that this region
would differ much from those of other hystricognath rodents. Large trophoblastic
cells invade an area of debris; they invade into and circle around maternal
vascular channels. This process of invasion has found great attention
by Nanaev et al. (1995) who showed clearly that it proceeds deeply into
the myometrium, primarily by encircling and then destroying the vascular
walls of the arteries. The muscular wall is destroyed but reconstitutes
quickly after parturition. In that respect it is similar to but not quite
identical with the extravillous trophoblast modification of human spiral
arterioles. The process is deemed to enhance blood flow by vascular dilatation.
NOS may play a major role in this process and was delineated in detail
by Nanaev et al. (1995).
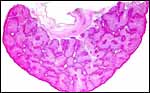
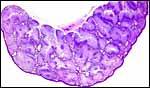
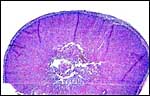
The ovary of pacaranas is distinctly unusual. By the time of the writing of the large review by Mossman and Duke (1984), it had not been described. The ovary is very lobulated (it has the appearance of a cerebral cortex) and is thus somewhat similar to that of a viscacha described by Weir (1971). It is also similar to the one viscacha gestation that I have been able to study from the San Diego Zoo. Whether pacaranas share any of the other unusual reproductive behaviors (such as the enormous number of eggs ovulated by the viscacha) needs to be studied. No endocrine studies have been undertaken.
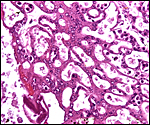
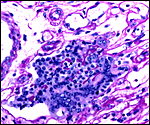
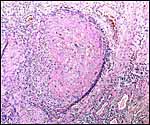
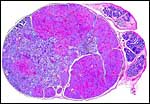
The histology of the fetal testis from the mature specimen discussed here is remarkable. It contained several large nodules of interstitial cells, in addition to the normally dispersed interstitial cell component. Three additional pacarana neonates were studied and all showed this massive interstitial cell proliferation; one is shown below. At this time it is unknown whether this is a normal feature, but it is certainly found in all neonates and fetuses available to us. Endocrine studies would be of great interest. In addition, the fetal adrenal is large and has a "fetal zone" of its cortex that has very much the appearance of human adrenal glands in newborns. Endocrine studies of this unusual species are equally urgently needed to understand the physiology of fetal life.
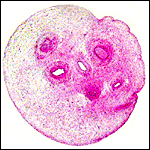
Pacaranas have 58 chromosomes. A karyotype of an animal studied at CRES (San Diego Zoo) is shown below. No other genetic studies are known to me.
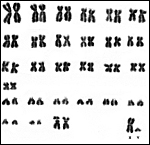 |
Karyotype of male pacarana, 2n=58. The sex chromosomes are shown last. The last pair of autosomes has prominent satellites. |
To my knowledge there are no reported studies. In view of the similarity of this placenta with other rodents' placentas and especially those of the hystricognaths, the probability of immunoglobulin transport through the inverted yolk sac membrane is highly likely. This was reviewed in some detail by King (1977).
15)
Pathological features
Animals in our colony have died from uremia, secondary to chronic nephritis
and obstructed ureters. Meritt (1984) reported the deaths of captive specimens
from heavy infestation with Strongyloides sp. Ascaridiasis and
seborrhea are other problems mentioned by this author. The lung of the
female whose post partum uterus is described above did not have the trophoblast
dissemination that has been observed in some viscacha specimens. Stillbirths
have occurred for unknown reasons, but they were also not attended, happening
at night. Dystocia led to Cesarean section in one animal. The area of
subplacental degeneration is often considered to be an "infarct",
when the observer is unfamiliar with that being a normal feature of these
rodents.
16)
Physiologic data
There is no knowledge other than what has already been summarized by Meritt
(1984), and by Collins & Eisenberg (1972).
17)
Other resources
Cell strains from many animals are available from CRES
at San Diego Zoo, by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
Much more information is needed on the general biology and especially
on the reproductive physiology of this unusual rodent. Endocrine parameters
are completely lacking. An implanted placenta is needed for detailed study.
Of great interest are the development of the subplacenta and the depth
of arterial infiltration of trophoblast.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the assistance by Professor
Dr. Hiroaki Soma of Tokyo for obtaining the immature placental specimen
described above. Pathological material was kindly made available by the
pathologists at the San Diego Zoo. The mature placenta and fetal organs
were kindly provided by Dr. Richard Montali of the National Zoo, Washington,
DC.
References
Collins, L.R. and Eisenberg, J.F.: Notes on the behaviour and breeding
of pacaranas Dinomys branickii in captivity. Intern. Zoo Yearbk.
12:108-114, 1972.
Luckett, W.P.: Monophyletic or diphyletic origins of anthropoidea and hystricognathi. Evidence of the fetal membranes. Chapter 17, pp. 347-368, in, Evolutionary Biology of the New World Monkeys and Continental Drift. R.L. Ciochon & A.B. Chiarelli, eds. Plenum Press, N.Y. 1980.
Luckett, W.P. and Mossman, H.W.: Development and phylogenetic significance of the fetal membranes and placenta of the African hystricognathous rodents Bathyergus and Hystrix. Amer. J. Anat. 162:265-285, 1981.
King, B.F.: An electronmicroscopic study of absorption of peroxidase-conjugated immunoglobulin G by guinea pig visceral yolk sac in vitro. Amer. J. Anat. 148:447-456, 1977.
Meritt, D.A.: The Pacarana, Dinomys branickii. Chapter 16, in: One Medicine, O.A. Ryder and M.L. Byrd, eds., Springer-Verlag, NY, 1984.
Miglino, M.A., Carter, A.M., dos Santos Ferraz, R.H. and Fernandes Machado, M.R.: Placentation in the capybara (Hydrochaerus hydrochaeris), agouti (Dasyprocta aguti) and paca (Agouti paca). Placenta 23:416-428, 2002.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nanaev, A., Chwalisz, K., Frank, H.-G., Kohnen, G., Hegele-Hartung, C. and Kaufmann, P.: Physiological dilatation of Uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res. 282:407-421, 1995.
Nguyen,
K.H.: Dinomys branickii, pacarana, false paca.
http://animaldiversity.ummz.umich.edu/accounts/dinomys/d_branickii
Roberts, C.M. and Perry, J.S.: Hystricomorph embryology. In, The Biology of the Hystricomorph Rodents. I.W. Rowlands & B.J. Weir, eds. Symp. Zool. Soc. London # 34: 333-360, 1974 (Academic Press, London).
Simpson, G.: Splendid Isolation. Yale University Press, New Haven, 1980.
Weir, B.J.: The reproductive organs of the female plains viscacha, Lagostomus maximus. J. Reprod. Fertil. 25:365-373, 1971.
Wilson, D.E. and Reeder, D.A.M.: Mammal Species of the World. A Taxonomic and Geographic Reference. 2nd ed. Smithsonian Institution Press, Washington, DC, 1992.