| |
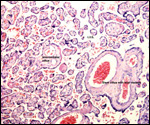
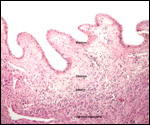
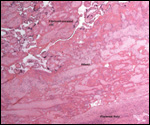
A variety of infectious diseases (pneumonia, nematodes) has been reported
(Scott, 1992). Diverticulitis, with fatal peritonitis, was recorded by Murray
et al. (2000) to have occurred in a 30 year-old Sumatran orangutan. Miyagi
et al. (1999) described death of a 37 year-old animal due to Coxsackie B4
myocarditis. Griner (1983) described the post partum death of an orangutan
due to "toxemia of pregnancy" (renal necrosis, proteinuria - same
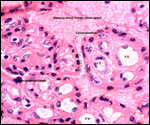
case as referred to above). Lowenstine (1986) referred to a mast cell tumor
of the eyelid, an ovarian granulosa cell tumor (with endometriosis), uterine
leiomyomas in orangutans, and an esophageal squamous cell carcinoma in a
35 year-old animal. Generally speaking though, neoplasms are uncommon.
16)
Physiological data
No relevant studies have been reported on orangutans.
17)
Other resources
Cell strains of numerous animals of both species and their hybrids are
available from CRES at San Diego Zoo.
18)
Other relevant features
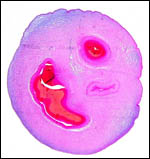
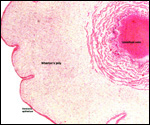
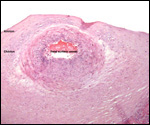
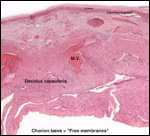
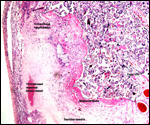
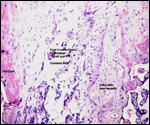
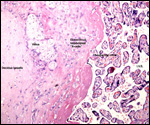
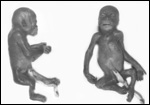
Twins are occasionally observed, and they may be delivered at term to
grow up, as did those associated with the twin placentas shown above.
Geissmann (1990) found a 1.1% twinning frequency (7 in 626 births) in
orangutans, not much different from other apes and man. No specific studies
as to zygosity have been reported. Fraternal twins (M/F) with a fused,
diamnionic dichorionic twin placenta were described by Heinrichs &
Dillingham (1970). The male weighed approximately 1,500 g, the female
was only about 900 g; they did well being hand-reared. The umbilical cords
were 40 and 45 cm long, with the former having a tight knot, that which
belonged to the smaller, female infant.
Schroeder et al. (1978) reported on the nature of fetal hemoglobin chains
of orangutans.
References
Andrle, M., Fiedler, W., Rett, A., Ambros, P. and Schweizer, D.: A case
of trisomy 22 in Pongo pygmaeus. Cytogenet. Cell Genet. 24:1-6,
1979.
Benirschke,
K.: Chorioamnionitis as cause of abortion in orangutan (Pongo pygmaeus
abeli). J. Zoo Anim. Med. 14:56-60, 1983.
Benirschke,
K. and Kaufmann, P.: Pathology of the Human Placenta. Springer-Verlag,
NY 2000.
Bowman,
M.E., Lopata, A., Jaffe, R.B., Golos, T.G., Wickings, J. and Smith, R.:
Corticotropin-releasing hormone-binding protein in primates. Amer. J.
Primatol. 53:123-130, 2001.
Cell
strains available from: http://www.sandiegozoo.org/conservation/frozen.html.
Cowlishaw,
G. and Dunbar, R.: Primate Conservation Biology. University of Chicago
Press, 2000.
Czekala,
N.M., Benirschke, K., McClure, H. and Lasley, B.L.: Urinary estrogen excretion
during pregnancy in the lowland gorilla (Gorilla gorilla), orangutan
(Pongo pygmaeus) and the human (Homo sapiens) Biol. Reprod.
28:289-294, 1983.
De
Boer, L.E.M. and Seuánez, H.N.: The chromosomes of the orangutan
and their relevance to the conservation of the species. In, Biology and
Conservation of the Orangutan, L.E.M. de Boer, ed. W. Junk, The Hague,
1982.
Dutrillaux,
B., Rethoré, M.O. and Lejeune, J.: Comparaison du caryotype de
l'orang-outang (Pongo pygmaeus) a celui de l'homme, du chimpanzé
et du gorille. Ann. Génét.: 18:153-161, 1975.
Gagneux,
P. and Varki, A.: Genetic differences between humans and great apes. Molec.
Phylogenet. Evol. 18:2-13, 2001.
Geissmann,
T.: Twinning frequency in catarrhine primates. Human Evol. 5:387-396,
1990.
Greenberg,
M.J., Janssen, D.L., Jamieson, S.W., Rothman, A., Frankville, D.D., Cooper,
S.D., Kriett, J.M., Adsit, P.K., Shima, A.L., Morris, P.J. and Sutherland-Smith,
M.: Surgical repair of a atrial septal defect in a juvenile Sumatran orangutan
(Pongo pygmaeus sumatrensis). J. Zoo & Wildl. Med. 30:256-261,
1999.
Graham-Jones,
O. and Hill, W.C.O.: Pregnancy and parturition in a Bornean orang. Proc.
Zool. Soc. London 139:503-510, 1962.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, 1983.
Hamerton,
J.L., Klinger, H.P., Mutton, D.E. and Lang, E.M.: The somatic chromosomes
of the Hominoidea. Cytogenetics 2:240-263, 1963.
Heinrichs,
W.L. and Dillingham, L.A.: Bornean orang-utan twins born in captivity.
Folia Primatol. 13:150-154, 1970.
Jones,
S., Martin, R. and Pilbeam, D. (eds.): The Cambridge Encyclopedia of Human
Evolution. Cambridge University Press, 1995.
Kenyon,
L. and Moraes, C.T.: Expanding the functional human mitochondrial DNA
database by the establishment of primate xenomitochondrial cybrids. Proc.
Natl. Acad. Sci. USA 94:9131-9135, 1997.
Kingsley, S.R. and Martin, R.D.: A case of placenta praevia in an orang-utan. Vet. Rec. 104:56-57, 1979.
Lindburg,
D.G., Berkson, J.M. and Nightenhelser, L.K.: Primate breeding in zoos:
A ten year summary. Chapter 17 (pp.162-170) in, One Medicine, O.A. Ryder
& M.L. Byrd, eds. Springer-Verlag, NY, 1984.
Lowenstine,
L.J.: Neoplasms and proliferative disorders in nonhuman primates, Chapter
53 (pp. 781-814), in, Primates - The Road to Self-sustaining Populations;
K. Benirschke, ed., Springer-Verlag, N.Y. 1986.
Maggioncalda,
A.N., Sapolsky, R.M. and Czekala, N.M.: Reproductive hormone profiles
in captive male orangutans: Implications for understanding developmental
arrest. Amer. J. Phys. Anthropol. 109:19-32, 1999.
Miyagi,
J., Tsuhako, K., Kinjo, T., Iwamasa, T., Kamada, Y., Kinju, T. and Koyanaga,
Y.: Coxsackievirus B4 myocarditis in an orangutan. Vet. Pathol. 36:452-456,
1999.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Myrray,
S., Zdziarski, J.M., Bush, M., Citino, S.B., Schulman, F.Y. and Montali,
R.: Diverticulitis with rupture and fatal peritonitis in a Sumatran orangutan
(Pongo pygmaeus) Comp. Med. 50:452-454, 2000.
Muir,
C.C., Galdikas, B.M. and Beckenbach, A.T.: Is there sufficient evidence
to elevate the orangutan of Borneo and Sumatra to separate species? J.
Molec. Evol. 46:378-379, 1998.
Muir,
C.C., Galdikas, B.M. and Beckenbach, A.T.: mtDNA sequence diversity of
orangutans from islands of Borneo and Sumatra. J. Molec. Evol. 51:471-480,
2000.
Naaktgeboren,
C. and v. Wagtendonk, A.M.: Wahre Knoten in der Nabelschnur nebst Bemerkungen
über Plazentophagie bei Menschenaffen. Z. Säugetierk. 31:376-382,
1966.
Panigel,
M.: Comparative anatomical, physiological and pharmacological aspects
of placental permeability and haemodynamics in the non-human primate placenta
and in isolated perfused human placenta. Pp. 279-295, 1968 in, The Foeto-Placental
Unit. Excerpta Medica Internat. Congress Series # 183.
Pepe,
G.J. and Albrecht, E.D.: Actions of placental and fetal adrenal steroid
hormones in primate pregnancy. Endocrine Reviews 16:608-648, 1995.
Puschmann,
W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag,
Berlin, 1989.
Ramsey,
E.M., Corner, G.W. and Donner, M.W.: Serial and cineradioangiographic
visualization of maternal circulation in the primate (hemochorial) placenta.
Amer. J. Obstetr. Gynecol. 86:213-225, 1963.
Ramsey,
E.M. and Harris, J.W.S.: Comparison of uteroplacental vasculature and
circulation in the rhesus monkey and man. Carnegie Inst. Publication 625,
Contributions to Embryology # 261. Vol. 38:59-70, 1966.
Rijksen,
H.D.: Conservation of orangutans: A status report, 1985. Chapter 10 (pp.
154-159) in, Primates - The Road to Self-sustaining Populations; K. Benirschke,
ed., Springer-Verlag, N.Y. 1986.
Ryder,
O.A. and Chemnick, L.G.: Chromosomal and mitochondrial DNA variation in
orang utans. J. Hered. 84:405-409, 1993.
Schroeder,
W.A., Shelton, J.R., Shelton, J.B. and Huisman, T.H.J.: The v? chain of
fetal hemoglobin of the orangutan. Biochem. Genet. 16:1203-1205, 1978.
Schwartz,
J.H.: The evolutionary relationships of man and orang-utans. Nature 308:501-505,
1984.
Scott,
G.B.D.: Comparative Primate Pathology. Oxford University Press, Oxford,
1992.
Seuánez,
H.N.: Chromosome studies in the orangutan (Pongo pygmaeus): practical
application for breeding and conservation. Zoo Biol. 1:179-199, 1982.
Seuánez,
H.N., Fletcher, J., Evans, H.J. and Martin, D.E.: A polymorphic structural
rearrangement in two populations of orangutans. Cytogenet. Cell Genet.
17:327-337, 1976.
Seuánez,
H.N., Evans, H.J., Martin, D.E. and Fletcher, J.: An inversion in chromosome
2 that distinguishes between Bornean and Sumatran orangutans. Cytogenet.
Cell Genet. 23:137-140, 1979.
Seuánez,
H.N.: Chromosomal and molecular characterization of the primates: Its
relevance in the sustaining of primate populations. Chapter 60 (pp. 887-910)
in, Primates - The Road to Self-Sustaining Populations; K. Benirschke,
ed., Springer-Verlag, N.Y. 1986.
Smith,
R.J. and Pilbeam, D.R.: Evolution of orang-utan. Nature 284:447-448, 1980.
Soma,
H.: Notes on the morphology of the chimpanzee and orang-utan placenta,
Placenta 4:279-290, 1983.
Spatz,
W.B.: Nabelschnur-Längen bei Insektivoren und Primaten. Z. Säugetierk.
33:226-239, 1968.
Stanyon,
R. and Chiarelli, B. Phylogeny of the Hominoidea: The chromosome evidence.
J. Hum. Evol. 11:493-504, 1982,
Strahl,
H.: Uteri gravidi des Orangutan. Anat. Anz. 22:170-175, 1903. (in German).
Templeton,
A.R.: The phylogeny of the hominoid primates: A statistical analysis of
the DNA-DNA hybridization data. Mol. Biol. Evol. 2:420-433, 1985.
Turleau
C., de Grouchy, J. and Klein, M.: Phylogénie chromosomique de l'homme
et des primates hominiens (Pan troglodytes, Gorilla gorilla
et Pongo pygmaeus). Essai de reconstruction du caryotype de l'ancêtre
commun. Ann. Génét. 15:225-240, 1972.
Warren,
K.S., Verschoor, E.J., Langenhuijzen, S., Heriyanto, S.R.A., Vigilant,
L. and Heeney, J.L.: Speciation and intrasubspecific variation of Bornean
orangutans, Pongo pygmaeus pygmaeus. Molec. Biol. Evol. 18:472-480,
2001.
Xu,
X. and Arnason, U.: The mitochondrial DNA molecule of Sumatran orangutan
and a molecular proposal for two (Bornean and Sumatran) species of orangutan.
J. Molec. Evol. 43:431-437, 1996.
|