|
(Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.)
|
Myocastor coypus
Order: Rodentia
Family: Myocastoridae
1) General Zoological Data
This South American rodent, a member of the Suborder Hystricomorpha, is widely distributed through Southern South America and, because of importations, it is now resident in Southern USA (Louisiana etc.), England, Poland, and many more countries which it did not inhabit originally. Several (regional) subspecies have been nominated and there is also a considerable coat color variety in the many colonies. The huge colony in Louisiana (millions) is said to have derived from only 13 ancestors, indicating the success that this animal has in some habitats. It has replaced many local species where it was introduced.
It might here be mentioned, however, that the term Hystricomorpha must be correctly applied and understood. The nomenclature was precisely defined by Simpson (1974) in his preliminary remarks to a symposium on the topic of Hystricomorpha. It is not, as he cautions, an evolutionary unit but an anatomical term. The animals reviewed here under that heading would thus be more correctly grouped as Caviomorpha. There has also been controversy regarding the placement under hystricomorphs. Thus, Lowery (1974) placed nutrias in the family Capromyidae. The most recent review by Wilson & Reeder (1992) separates nutria as the only member of the Myocastoridae. They discuss briefly the reasons for separating this species from other South American rodents. The name Myocastor derives from the Greek mouse and beaver, while Coypu is an old South American native name (Gotch, 1979). It is said to mean "water-sweeper" (Lowery, 1974).
Coypus are extremely well adapted to water, are excellent swimmers, and prefer to feed in swampy areas off roots and other vegetable matter. Their nostrils are "valvular" and thus adapted for under-water swimming (Kinler et al., 1987). They also have been well-adapted to form large breeding colonies that are now widely exploited for fur production (Federspiel, 1941). Adults weigh between 3-14 kg (Hayssen et al., 1993), males being larger. Neonates weigh between 100 and 250 g; Newson (1966) gives a mean of 225 g for neonates. There are commonly around five young born, but variations occur, with as many as 13 animals in a litter (Newson, 1966). Prenatal demise is common in this species as well as in other hystricomorphs, and scars may be found of the uterus subsequently. On the other hand, on occasion fewer corpora lutea have been identified than there are implantations sites, thus leading to the speculation that occasional monozygotic offspring may occur. No direct evidence has been provided for this. A major reason for mortality is cold weather and frost to which these South American rodents are ill-adapted.
The chronology of pre-implantation stages in coypu was described by Felipe et al. (2002) from their daily colposcopic and cytological examinations. Flushing uteri yielded embryos to day 10 post coitus. Cleavage stages were observed from 3-6 days, morulae from 6-9 days, and blastocysts were obtained after day 8. Tubal specimens (morulae) were discovered that had up to 30 cells. The length of gestation is around 130 days (128-135). Two litters are usually born annually. Newson (1966) has reviewed the general reproductive physiology of the coypu in its British feral state and showed the growth rates of embryos, which is depicted in the next graph.
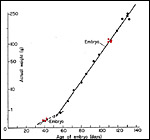 |
Fetal weights (Modified from Newson, 1966). Red dots represent gestational ages of animals used for this text. |
 |
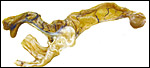
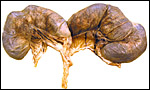
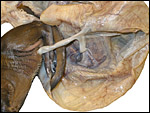
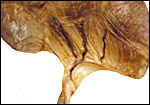
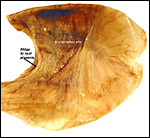
Term, delivered nutria with uterus still attached. |
The anatomy of nutria genital systems, both male and female, is comprehensively covered by Hillemann et al. (1958). They also reviewed older publications and the anatomy of genitalia from some related taxa. The publication shows excellent drawing of the genitalia. Males and females have bacula, although the female's is cartilaginous. The uterus is duplex with two cervical openings; the ovaries are not enclosed in a bursa. Implantation of the placental disk is mesometrial but the initial nidation is anti-mesometrial.
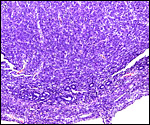
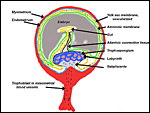
Hystricomorph rodents have a complex, interstitial implantation and placental development may be difficult to understand at first. Moreover, they are characterized by having a sizeable "subplacenta", the exact function of which remains to be elucidated. Other rodents lack this structure. In addition, they possess, as do other rodents, an inverted yolk sac through which proteinaceous substances are exchanged. All of these features make this a difficult organ to comprehend, especially as it changes its appearance with advancing gestational age.
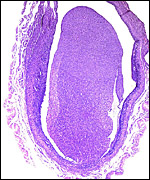
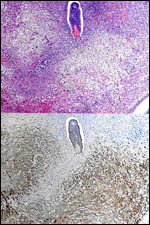
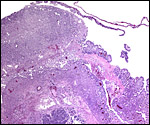
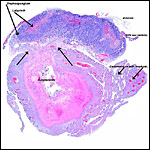
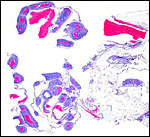
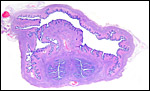
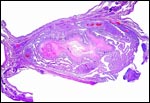
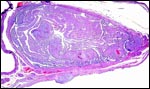
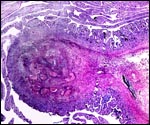
The early stages of placental development are complex and shown here in a series of photographs from successive stages. Implantation in rodents is preceded by significant decidualization of the endometrium, effected through progesterone and estrogen. It is complex and has been extremely well reviewed in great detail by Abrahamsohn & Zorn (1993). Although these stages have not been described for the nutria, one may assume that some of the processes are similar to other rodents, but I remain cautious at the same time.
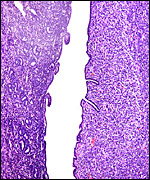
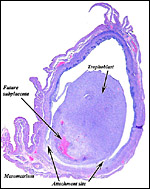
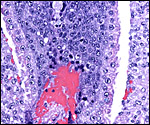
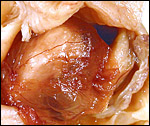
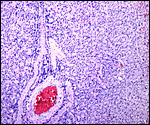
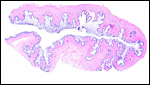
The blastocyst implants (interstitially) in a greatly expanded mass of decidualized endometrium. This initial attachment occurs anti-mesometrially. Once the trophoblast makes contact with the endometrial epithelium and penetrates it, trophoblast cells infiltrate this large mass of endometrium shown in one of the next photographs. The trophoblast then also replaces some of the endometrium, spreads laterally and attaches (implanted in endometrium) on a very narrow pedicle that is also shown here. Older authors have spoken of a "double implantation", as there is initial attachment anti-mesometrially but the disk develops mesometrially. Clefts develop in the endometrium so that, essentially, a second endometrial cavity is created that thus explains the band-like extensions seen in the photographs.
The juxtaposition of trophoblast to the endometrial surface and also its invasion into the decidua, in irregular columns, are shown next.
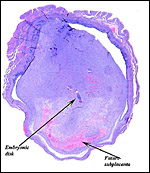
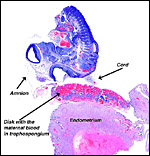
From the ectoplacental region of the blastocyst trophoblastic cells have pierced the single-layered endoderm and grow towards the decidual portion of the mesometrial region, thus establishing the precursor of the future mesometrial placental disk. The embryo is always located towards the future region of the disk, away from the original anti-mesometrial implantation site.
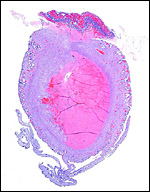
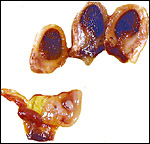
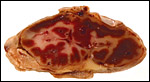
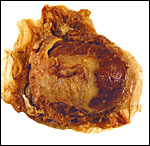
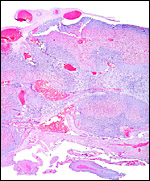
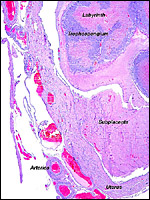
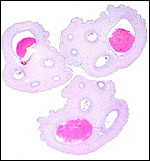
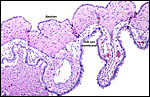
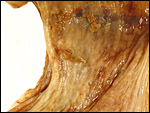
The nutria placenta is discoid, labyrinthine and hemomonochorial with a prominent subplacenta. It is mesometrially implanted in the duplex uterus. An excellent and comprehensive description of the advanced stages of the nutria placenta has been provided by Hillemann & Gaynor (1961). Their publication included specimens that were injected with various substances in order to obtain a better understanding of the maternal and fetal vasculatures. It also includes measurements and weights of the disks. "In the full-term gestation sac, the uterine epithelium completely lines the uterine lumen, except for the very restricted area occupied by the delicate and tenuous vessels of the basal plate", they stated. "The yolk sac placenta is solely visceral but highly developed. It forms a tough reinforcing investing membrane on the outside of the amnion. It attaches to the outside of the amnion."
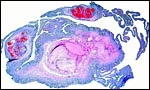
I have had the great fortune to obtain four gravid uteri at different stages of gestation. The largest uterus had five fetuses weighing between 140 and 155 g and measuring 12-13 cm CR length. Their placentas weighed (detached) 20-23 g and measured 4 x 1.2 cm. The cross sections of the disks show the characteristic pattern seen also in other South American rodents (see chapters on Porcupine and Pacarana).
In the other uteri of younger gestations obtained, the embryos were too small to be weighed, but measured 1.2 - 1.4 cm in CR length.
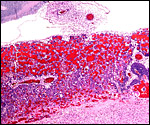
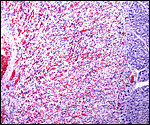
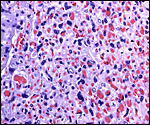
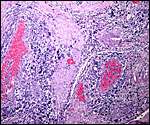
The labyrinth comprises the feta/maternal barrier through which most nutrients and gases are exchanged. Here, thin blood channels and trophoblastic tubes (containing maternal blood) extend from the center to the trophospongium. Capillary anastomoses were described to exist here by Hillemann & Gaynor (1961) through their injection studies. These vascular channels are essentially composed of endothelial cells and most of the exchange would have to take place here. There is little connective tissue. The trophoblast is syncytial and extremely thin. Fine structural details of this complex labyrinth are only available for the chinchilla placenta (King & Tibbitts, 1976). It is likely, however, that it is very similar if not identical in the nutria. That publication described the maternal blood-containing channels or tubes as being lined, as it were, by syncytiotrophoblast. This, in turn, connects with fetal capillary endothelium or cytotrophoblast that can also be found here. The electron microscopic study of the chinchilla placenta by King& Tibbitts (1976) shows these cells very clearly.
The trophospongium consists mostly of syncytiotrophoblast and some sheaths of solid trophoblast, especially at the base. There, some giant cells are also found which, otherwise, cover most of the outside of the disk and are penetrated by a fine vascular meshwork.
The umbilical cord of the largest fetus shown here measured 3 cm in length and had only a minimal spiral. It was always looped around the fetus' body. I assume that it grows as the fetus lengthens from stretch imposed upon it. Hillemann & Gaynor (1961) measured the umbilical cord at term gestation to be 6.5 x 0.45 cm in size. It branches near its insertion on the placental surface; the chorioallantoic portion then was 1 cm long, the vitelline portion was less than 0.5 cm and this branched again almost immediately into the numerous smaller vessels of the vitelline membrane. The amnionic surface of the cord was smooth and composed of a single-layered squamous epithelium. The complete cord had five blood vessels; the chorioallantoic part had three vessels, and the vitelline part contained only two blood vessels. There are no ducts and no smaller vessels are present as seen for instance in ungulates. The surface is covered with amnion which has no squamous plaques.
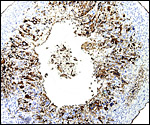
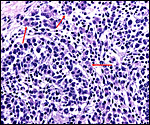
Hillemann & Gaynor (1961) provided a most detailed description of the complex vasculature with the help of their injection studies. They found no endovascular "plasmodia" (I interpret this to mean syncytiotrophoblast). My preparations, however, show that not only is trophoblast growing within the maternal blood vessels but it surrounds in great numbers the maternal vasculature of the myometrium and the mesometrium as well. In many vessels it replaces the muscular wall completely. Moreover, both, arteries and (less so the) veins are similarly infiltrated by trophoblast, as will be seen in the photographs below; nevertheless, the veins have much less trophoblastic investment than the arteries. The trophoblast is strongly cytokeratin positive and resembles closely the picture shown in the pacarana (see Chapter on Pacarana).
A very similar picture is found in the guinea pig, and it is perhaps characteristic of Caviomorpha in general (see Nanaev et al., 1995). Indeed, Kaufmann (personal communication) informed me that one can observe this peritoneal trophoblastic infiltration when opening the abdomen of a pregnant caviomorph. There is a perceptible thickening in the size of mesometrial vessels in those vessels that go to the disks, not in the remainder. Their Figure 1 illustrates this aspect superbly. The complex perfusion of the labyrinth and trophospongium was illustrated for the guinea pig in diagrams by Kaufmann (1981). It is the same in nutria.
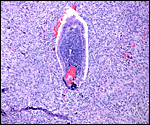
Luckett (1980) suggested that most caviomorph rodents studied form the amnionic cavity by folding, a point also supported by Roberts & Perry (1974). He also further described the vitelline placental formation of Caviomorpha. The membranous dome of the placental sac is extremely thin. It consists first of the peripheral uterine muscle, then endometrium, finely vascularized yolk sac membrane with thin epithelium, and finally the avascular amnion. At the interface to the next placenta, a significant ridge is formed that is shown in the next photograph. The amnionic cavity contained practically no amnionic, especially in the largest gestations available to me. The vitelline membrane remains very well preserved throughout gestation and, in analogy with other Rodentia, one would assume that some exchange between mother and fetus (immunoglobulins, other proteins) may be take place here (see King, 1977).
Trophoblast invades the uterus deeply and follows maternal myometrial arteries which it envelops in large numbers. This is not unlike the condition found in some other South American rodents and especially the hystricomorpha (see Chapters on Pacarana, American Porcupine, Beaver; and Nanaev et al., 1995). These cells have a very varied appearance; most are unusually large, many appear to be polyploid, have huge nuclei and large nucleoli. In addition, syncytial cells are present in this mantle of trophoblast around the maternal blood vessels.
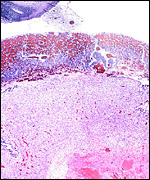
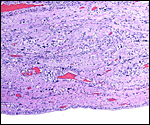
The remarkable finding in this and some other hystricomorphs is the relatively narrow pedicle that connects the subplacenta to the uterine musculature. Significant decidualization of the endometrium occurs early in gestation and becomes less prominent later. The extensive review of rodent decidualization by Abrahamsohn & Zorn (1993) needs to be consulted to fully comprehend the complex development of rodent decidua. Some trophoblast can be seen to infiltrate the endo- myometrium lateral to the implanted disk. Near the end of gestation the entire uterine cavity is lined by a thin endometrium without glands over the dome of gestational sacs.
Coypus have a pronounced subplacenta beneath the single disk and below it a "junctional zone" of debris. They are penetrated by small maternal blood vessels, the larger ones passing at the periphery of the subplacenta. The subplacenta consists of large numbers of trophoblastic cells, including giant cells, below which is much fibrin and degenerated material, including some foci of calcification. Scattered trophoblast and giant cells are present within the subplacental debris. Its function has been debated for a long time and is not yet completely resolved. Mossman (1987) considered it extensively. He maintained that it had to represent a zone of growth, a "germinative region", for placental expansion. A secretory function was assigned to this region (in the guinea pig placenta) by the fine structural studies of Wolfer & Kaufmann (1980). They considered the region to be metabolically highly active but not an area of fetal/maternal exchange. Others have speculated that it might be the source of "PBG" (progesterone binding protein - see subsequent section on Endocrinology).
12) Endocrinology
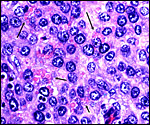
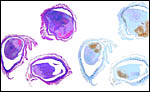
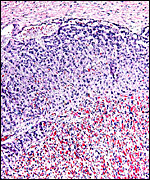
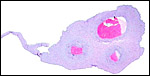
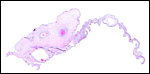
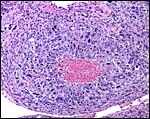
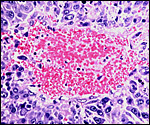
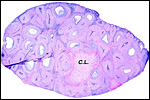
The maternal ovary is small and ovoid, with scattered corpora lutea and
a plethora of luteinized follicles. They are not folded as in Pacarana
or Chinchilla. Appreciably more luteinization of Graafian follicles is
found as gestation advances. An extensive description of the ovaries can
be found by Rowlands & Heap (1966). Felipe et al. (1999) have also
described them in great detail. The ovaries of both, fetuses and adults,
are located remarkably close to the kidneys. The fetal adrenal glands
are diminutive. Weir & Rowlands (1974) depicted an essentially similar
ovary as that shown below of late pregnancy with numerous luteinized follicles.
The seemingly excessive luteinization of the follicles of hystricomorph
rodents during pregnancy is perhaps one means of prolonging the gestation
in these unusual animals, as Amoroso (1974) suggested in his highly readable
summarizing remarks to that symposium already mentioned. There is no ovarian
bursa. The fetal testes of the specimens I was able to study had only
a moderately active population of interstitial cells, both in young and
the older embryos. The fetal ovaries showed no endocrine stimulation whatever.
There is a dramatic rise in progesterone in mid-pregnancy and a fall some
weeks before parturition, perhaps coincident with follicular luteinization.
Rowlands & Heap (1966) speculated that the luteotropic activity may
derive from placental secretions but no direct evidence was then presented.
Since the coypu, as the guinea pig, is a member of the larger group of
Hystricomorpha, these authors suggested that similar mechanisms
of the maintenance of gestation may prevail. In guinea pigs, pregnancy
continues when, in later gestation, the ovaries are removed, suggesting
that placental hormone (?progesterone) secretion is sufficient for the
endocrine support of gestation. This was not the case with coypu. When
their ovaries were removed during gestation, the pregnancies aborted.
Wang et al. (1990) experimented with passive immunization of mice with a monoclonal progesterone antibody (B3) and found it to block pregnancy in a number of species. It was found in uterine lumens 36 hours after injection and it binds also to progesterone in the DB3-protein of coypu.
The morphologic aspects of uterus, cervix and Fallopian tube were described in detail by Felipe et al. (1998), and an excellent description of the gross anatomy of both sexes can also be found by Hillemann et al. (1958).
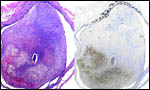
Nutrias have 42 chromosomes, all metacentrics with the exception of the Y chromosome (Fredga, 1966; Tsigalidou et al., 1968). I am not aware that coypu have hybridized with other rodents, and no hybrids are mentioned by Gray (1972). Garcia-Meunier et al. (2001) prepared a technique of PCR amplification of the Sry gene in order to sex coypu embryos efficiently. There is considerable color variation of nutrias within breeding colonies, an aspect that affects marketability (Kinler et al., 1987). These authors also give reference to some protein polymorphisms that have been identified.
 |
Karyotypes of male and female nutrias. |
I am not aware of any immunological studies that have been published on coypu. It would be an interest, however, to learn more about the MHC situation in all hystricomorphs, as there is such extensive infiltration of trophoblast into the maternal vasculature and exposure of fetal antigens to the maternal immune system should prevail. Enders & Welsh (1993) have speculated on the mechanisms that might prevent "rejection" of placentas. A consideration of this aspect, however, is deemed to be inappropriate here.
15)
Pathological features
Epizootic pneumonia has become a major disease of nutria and was studied
by Martino & Stanchi (1994) in Argentina. They considered that Streptococcus
zooepidemicus was a major cause, although other organisms (but no
viruses) were also isolated in pneumonia cases. Howerth et al. (1994)
studied some exposure to diseases in coypu that had been trapped in Louisiana.
They found sporadic antibodies against toxoplasma, chlamydia, and leptospira,
but none against tularemia and encephalomyocarditis virus. Giardia were
isolated in 22 of 30 nutria captured in East Texas by Dunlap & Thies
(2002). Griner (1983) observed endometrial polyps, metritis, nephritis,
and hemopericardium from an aortic rupture. Kinler et al. (1987) make
reference to the frequent dermatitis (due to a tick) and other parasites
affecting nutria.
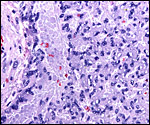
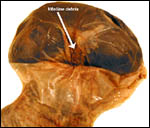
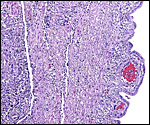
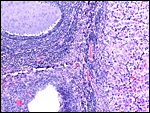
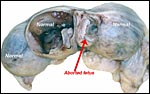
As in some other rodent gestations, there are occasional fetal/embryonic
demises that are readily manifest macroscopically by a smaller than expected
bulge of the uterine horn. One such fetal death is shown in the next few
pictures.
Bo et al. (1994) have described the techniques for immobilization of copy with ketamine and xylazine. The structure of coypu insulin was determined by Smith (1972). Jelinik & Glasrova (1982) described the red cell findings in postnatal coypu and other biochemical studies are referred to in Kinler et al. (1987).
17)
Other resources
Cell lines of several nutria at CRES
of the San Diego Zoo can be made available through contacting Dr. O. Ryder
at
oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
Immunological studies are lacking and especially the MHC gene expression
during gestation would be of interest because of the massive trophoblast
infiltration into the uterus. More endocrine studies of early pregnancy
and, especially, insight into whether the placenta produces hormones would
be of interest.
Acknowledgement
The animal pictures and tissues were kindly made available to me through
the generosity of Dr. Val Lance (CRES), and Ruth M. Elsey, Louisiana Department
of Wildlife & Fisheries, Rockefeller Wildlife Refuge, Grand Chenier,
Louisiana.
References
Abrahamsohn, P.A. and Zorn, T.M.T.: Implantation and decidualization in
rodents. J. Exp. Zool. 266:603-628, 1993.
Amoroso, E.C.: Concluding remarks. In, "The Biology of Hystricomorph Rodents", I.W. Rowlands and B.J. Weir, Eds. Pp.447-453. Symposia Zoological Society London, # 34. Academic Press, 1974.
Bo, R.F., Palomares, F., Beltran, J.F., de Villafane, G. and Moreno, S.: Immobilization of coypus (Myocastor coypus) with ketamine hydrochloride and xylazine hydrochloride. J. Wild. Dis. 30:596-598, 1994.
Dunlap, B.G. and Thies, M.L.: Giardia in beaver (Castor canadensis) and nutria (Myocastor coypus) from East Texas. J. Parasitol. 88:1254-1258, 2002.
Enders, A.C. and Welsh, A.O.: Structural interactions of trophoblast and uterus during hemochorial placenta formation. J. Exp. Zool. 266:578-587, 1993.
Federspiel, M.N.: Nutria farming. Amer. Fur Breeder 13:12-13, 1941.
Felipe, A., Callejas, S. and Cabodevila, J.: Anatomicohistological characteristics of female genital tubular organs of the South American nutria (Myocastor coypus). Anat. Histol. Embryol. 27:245-250, 1998.
Felipe, A., Cabodevila, J. and Callejas, S.: Anatomicohistological characteristics of the ovary of the coypu (Myocastor coypus). Anat. Histol. Embryol. 28:89-95, 1999.
Felipe, A.E., Teruel, M.T., Cabodevila, J.A. and Callejas, S.S.: Pre-implantational timetable of embryonal development of Myocastor coypus (Coypu). Reprod. Nutr. Dev. 42:15-24, 2002.
Fredga, K.: Chromosome studies in five species of South American rodents (Suborder Hystricomorpha). Mammal. Chromosom. Newsl. #20:45, 1966.
Garcia-Meunier, P., Pastout, L., Chevalier, G. and Guinet, C.: Rapid determination of sex in Myocastor coypus embryos in the first stage of gestation. C.R. Acad. Sci. III 324:321-325, 2001.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca, 1993.
Heap, R.B. and Illingworth, D.V.: The maintenance of gestation in the guinea-pig and other hystricomorph rodents: Changes in the dynamics of progesterone metabolism and the occurrence of progesterone-binding globulin (PBG). In, "The Biology of Hystricomorph Rodents", I.W. Rowlands and B.J. Weir, Eds. Pp.395-415. Symposia Zoological Society London, # 34. Academic Press, 1974.
Hillemann, H.H., Gaynor, A.I. and Stanley, H.P.: The genital systems of nutria (Myocastor coypus) Anat. Rec. 130:515-531, 1958.
Hillemann, H.H. and Gaynor, A.I.: The definitive architecture of the placentae of nutria, Myocastor coypus (Molina). Amer. J. Anat. 109:299-317, 1961.
Howerth, E.W., Reeves, A.J., McElveen, M.R. and Austin, F.W.: Survey for selected diseases in nutria (Myocastor coypus) from Louisiana. J. Wildl. Dis. 30:450-453, 1994.
Jelinik, P and Glasrova, M.: The red blood picture in male coypu in the postnatal period. Vet. Med. 27:337-348, 1982). In Czech, with English summary.
Kaufmann, P.: Functional anatomy of the non-primate placenta. Placenta. (Suppl. 1. "Transfer Across the Primate and Non-Primate Placenta, Chapter 2) 13:-28, 1981.
King, B.F.: An electron microscopic study of absorption of peroxidase-conjugated immunoglobulin G by guinea pig visceral yolk sac in vitro. Amer. J. Anat. 148:447-456, 1977.
King, B.F. and Tibbitts, F.D.: The fine structure of the chinchilla placenta. Amer. J. Anat. 145:33-56, 1976.
Kinler, N.W., Linscombe, G. and Ramsey, P.R.: Nutria. Chapter 27, pp.326-343. In, Wild Furbearer Management and Conservation in North America. M. Novak, J.A. Baker, M.E. Obbard and B. Malloch, eds. Ontario Ministry of Natural Resources. Ashton-Potter, Ldt., Concord, Ontario, Canada, 1987.
Lowery, G.H., Jr.: The Mammals of Louisiana and its adjacent Waters. Louisiana State University Press, Baton Rouge, LA, 1974.
Luckett, W.P.: Monophyletic or diphyletic origins of anthropoidea and hystricognathi. Evidence of the fetal membranes. Pp. 347-368, in, Evolutionary Biology of the New World Monkeys and Continental Drift. R.L. Ciochon and A.B. Chiarelli, eds. Plenum Press, NY. 1980.
Martino, P. and Stanchi, N.: Epizootic pneumonia in nutria. Zbl. Veterinärmed. (B) 41:561-566, 1994.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nanaev, A., Chwalisz, K., Frank, H.-G., Kohnen, G., Hegele-Hartung, C. and Kaufmann, P.: Physiological dilatation of Uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res. 282:407-421, 1995.
Newson, R.M.: Reproduction in the feral coypu (Myocastor coypus). In, "Comparative Biology of Reproduction in Mammals", I.W. Rowlands, Ed. Pp.323-334. Symposia Zoological Society London, # 15. Academic Press, 1966.
Peel, S.: Granulated metrial gland cells. Adv. Anat. Embryol. Cell Biol. 115:1-112, 1989.
Roberts, C.M. and Perry, J.S.: Hystricomorph embryology. In, "The Biology of Hystricomorph Rodents", I.W. Rowlands and B.J. Weir, Eds. Pp333-360. Symposia Zoological Society London, # 34. Academic Press, 1974.
Rowlands, I.W. and Heap, R.B.: Histological observations on the ovary and progesterone levels in the coypu, Myocastor coypus. In, "Comparative Biology of Reproduction in Mammals", I.W. Rowlands, Ed. Pp.335-352. Symposia Zoological Society London, # 15. Academic Press, 1966.
Schally, A.V., Itoh, Z., Carter, W.H., Saito, M., Sawano, S., Arimura, A. and Bowers, C.Y.: Effect of luteinizing hormone-releasing factor (LRF) on plasma LH levels in nutria (Myocastor coypus). Gen. Comp. Endocr. 12:176-179, 1969.
Simpson, G.G.: Chairman's introductory remarks. In, "The Biology of Hystricomorph Rodents", I.W. Rowlands and B.J. Weir, Eds. Pp.1-5. Symposia Zoological Society London, # 34. Academic Press, 1974.
Smith, L.F.: Amino acid sequence of insulins. Diabetes 21 Suppl.2:467-460, 1972.
Tsigalidou, V., Simotas, A.G. and Fasoulas, A.: Chromosomes of the coypu. Nature 211:994, 1966.
Wang, M.W., Whyte, A., Heap, R.B. and Taussig, M.J.: Pregnancy-blocking progesterone antibody targets specifically the uterus through its progesterone-binding sites. J. Mol. Endocrinol. 4:283-291, 1990.
Weir, B.J. and Rowlands, I.W.: Functional anatomy of the hystricomorph ovary. In, "The Biology of Hystricomorph Rodents", I.W. Rowlands and B.J. Weir, Eds. Pp.303-332. Symposia Zoological Society London, # 34. Academic Press, 1974.
Wilson, D.E. and Reeder, D.A.M.: Mammal Species of the World. A Taxonomic and Geographic Reference. 2nd ed. Smithsonian Institution Press, Washington, DC, 1992.
Wolfer, J. and Kaufmann, P.: Die Ultrastruktur der Meerschweinchen-Subplazenta. Zbl. Vet. Med. C. Anatomia, Histologia, Embryologia. 9:29-43, 1980.