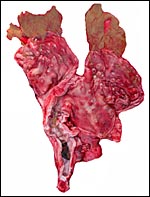
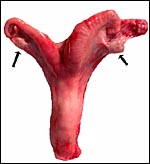
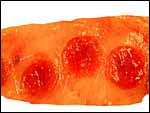
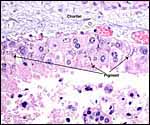
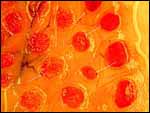
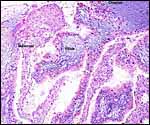
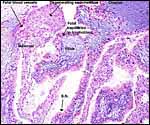
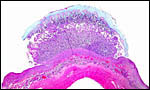
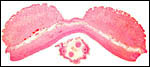
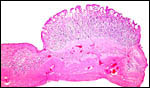
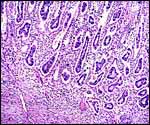
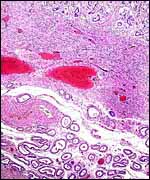
| |
17)
Other resources
Cell strains of numerous animals are available from CRES at the Zoological
Society of San Diego by requesting them from Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
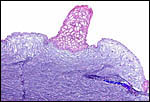
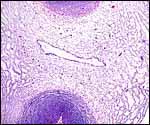
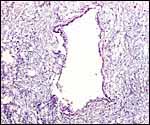
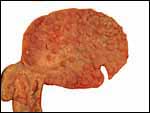
Endocrine data are virtually lacking and there is no description of implantational
stages. The comparison of the morphology and other aspects of physiology,
genetics, etc. suggests a closer relationship to the okapi than is commonly
appreciated (see the okapi chapter).
Acknowledgement
I appreciate very much the help of the veterinarians of the King Ranch
in Kingsville, Texas.
References
Beintema, J.J.: Primary structures of pancreatic ribonucleases from Bovidae,
Impala, Thomson's gazelle, nilgai and water buffalo. Biochem. Biophys
Acta. 252:89-103, 1980.
Benirschke,
K. and Kumamoto, A.T.: Mammalian cytogenetics and conservation of species.
J. Hered. 82187-191. 1991.
Blake,
J.E., Nielsen, N.O. and Heuschele, W.P.: Lymphoproliferation in captive
wild ruminants affected with malignant catarrhal fever: 25 cases (1977-1985).
J. Am. Vet. Med. Assoc. 196:1141-1143, 1990.
Chakraborty,
A.: Occurrence and pathology of Gongylonema infection in captive wild
herbivores. Vet. Parasitol. 52:163-167, 1994.
Davey,
.B.: Stagewise mortality, ovipositional biology, and egg viability of
Boophilus annulatus (Acari: Ixodidae) on Boselaphus tragocamelus
(Artiodactyla: Bovidae). J. Med. Entomol. 30:997-1002, 1993.
Erken,
A.H. and Wolters, S.A.: A case of possible rumenitis in a nilgai. Tijdschr.Diergeneeskd.104:57,
1979. (in Dutch).
Furley,
C.W., Taylor, W.P. and Obi, T.U.: An outbreak of peste des petits ruminants
in a zoological collection. Vet. Rec. 121:443-447, 1987.
Gallagher,
D.S. Jr., Davis, S.K., De Donato, M., Burzlaff, J.D., Womack, J.E., Taylor,
JF. and Kumamoto, A.T: Karyotypic analysis of nilgai, Boselaphus tragocamelus
(Artiodactyla; Bovidae). Chromosome Res. 6:505-5, 1998.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego,
California, 1983.
Hagey,
L.R., Gavrilkina, M.A. and Hofmann, A.F.: Age-related changes in the biliary
bile acid composition of bovids. Canad. J. Zool. 75:1193-1201, 1997.
Hassanin,
A. and Doucery, E.J.: The tribal radiation of the family Bovidae (Artiodactyla)
and the evolution of the mitochondrial cytochrome b gene. Mol. Phylogenet.
Evol. :227-243, 1999.
Jones,
M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169,
1993.
Matthee,
C.A. and Davis, S.K.: Molecular insights into the evolution of the family
Bovidae: a nuclear DNA perspective. Mol. Biol. Evol. 18:1220-1230, 2001.
Modi,
W.S., Gallagher, D.S. and Womack, J.E.: Evolutionary histories of highly
repeated DNA families among the Artiodactyla (Mammalia). J. Mol. Evol.
42:337-349, 1996.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Otcenasek,
M., Adamkova, A., Janeckova, V., Dvorak, J., Lavicka, M. and Micek, B.:
Dermatomycosis of the nilgai antelope (Boselaphus tragocamelus)
caused by the dermatophyte Trichophyton mentagrophytes. Vet. Med.
(Praha) 23:377-383, 1978. (In Czech).
Peinado,
V.I., Celdran, J.F. and Palomeque, J.: Basic hematological values in some
wild ruminants in captivity. Comp. Biochem. Physiol. A Mol. Integr. Physiol.
124:199-203, 1999.
Pepin,
L., Amigues, Y., Lepingle, A., Berthier, J.L., Bensaid, A. and Vaiman,
D.: Sequence conservation of microsatellites between Bos taurus
(cattle), Capra hircus (goat) and related species. Examples of
use in parentage testing and phylogeny analysis. Heredity 74:53-61, 1995.
Petit,
P., Vermeesch, J.R., Marynen, P. and De Meurichy, W.: Comparative cytogenetic
study in the subfamily Tragelaphinae. Proc. 11th Europ. Coll. Cytogenet.
Domest. Anim. Pp. 109-113, 1994.
Priebe,
J.C. and Brown, R.D.: Protein requirements of subadult nilgai antelope.
Comp. Biochem. Physiol. A 88:495-501, 1987.
Rajan,
A., Gangadharan, B. and George, P.O.: Intestinal diverticulitis in a nilgai
(Bucephalus tragocamelus). Vet. Rec. 135:626, 1994.
Sheffield,
W.J., Fall, B.A. and Brown: The nilgai antelope in Texas. Kleberg Studies
in Natural Resources. College Station, Texas: Texas Agricultural Experiment
Station,1983.
Vrba,
E.S. and Schaller, G.: Antelopes, Deer, and Relatives. Fossil record,
behavioral ecology, systematics, and conservation. Yale University Press,
New Haven, 2000.
|