|
(Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.)
|
| Last updated: October 30, 2004. |
Nasua narica yucatanica
Order: Carnivora
Family: Procyonidae
General Zoological Data
"There is only one species of coati, Nasua nasua…" (Simpson, 1980). Nowak (1999), however, accepted two species of coatis, the more northern species Nasua narica and the more southern South American species, Nasua nasua. Several subspecies have been alluded to as well, as far instance the species considered here that comes from Mexico. More southern South American coatis are a darker red than the browner northern animals. All have white noses, a feature often referred to. As the name suggests, the animal is characterized by its long, flexible nose and has a long, striped tail held upright most of the time. Coatis extend from Arizona (where they have become uncommon now) to Argentina and prefer wooded areas. They frequent trees, are mostly frugivorous but also hunt small insects, pray on eggs and some rodents. Beisiegel (2001) described the structure and behavior of coatis in Southern Brazil and related their preference for bromeliads. Adult coatis weigh up to 6 kg, while newborns are 100 g or more. Jones (1982) described their longevity as being at least 17 + years. The name "coatimundi" derives from the Tupi Indian name of this species (Gotch, 1979). Many zoos exhibit and breed coatimundis. Timock & Vaughan (2002) made a census of Cebus, coati and armadillo in the Punta Leona Refuge of Costa Rica and suggested from the data the population density. They sighted 148 cebus monkeys, 46 coatis and 8 nine-banded armadillos among some minor numbers of other species. Gompper et al. (1998) used multilocus DNA fingerprints to study the philopatry of coatis; females are more philopatric than males and close relationships exist in specific bands of animals. In a detailed study of mtDNA by Zhang & Ryder (1993) it was found that the lesser panda has no close relationship to either bears or procyonids; also, that the procyonids diverged from one another early although grouping together amongst the carnivores.
 |
Yucatan white-nosed coatimundi at San Diego Zoo. |
The length of gestation is 10-11 weeks (67-73 days) (Nowak, 1999) and produces between 2 and 7 young that weigh between 100 and 180 g. The placenta of the coatimundi is very similar to that of the raccoon that was well described by Watson (1881 ) and by Vacek (1951), but that publication was not accessible to me. It is zonary and, when delivered, it has an ovoid shape. In 1986 I had access to one full term coatimundi placenta that was still attached to a male neonate weighing 98 g. Its CR length was 12 cm and the tail measured 10.5 cm. This male neonate died from cryptosporidiosis. The placenta was ovoid and measured 5 x 2 cm. At one pole the placenta had a distinct yellow discoloration that is seen histologically as being represented by yellow crystalline deposits and appears to be the remains of the so-called haemophagous organ.
3) Implantation
No early stages of coati placentation have been observed. Moreover, no really
early raccoon placentas have as yet been described. These are important
aspects to be studied in the future. Mossman (1987) summarizes the procyonid
implantation to be "central", the yolk sac orientation to be mesometrial,
nidation to be superficial, and amniogenesis as occurring by folding.
4) General Characterization of the Placenta
The placenta of coatimundis has not been described previously. But several
studies of the related Raccoon (Procyon lotor) have been made,
with a most detailed first description by Watson (1881); several authors
have added to our knowledge since. The placenta of the coati is similar
in most respects to that of the raccoon. The implanted singleton raccoon
fetus (covered by a delicate "epitrichium") described by Watson
had a CR length of 4½ inches. It had the head facing the lateral
aspect of the uterus, towards the tubal end; the tail was situated between
the legs. The (detached) placenta was completely zonary ("annular")
and measured 1 ¼ inches in width. A very narrow zone of membranes
was described as being present in the circle and there was a smooth border
at the edge of the placenta due to attached decidua capsularis. Biggers
& Creeds (1962), however, found no interruption of the zonary nature
of the raccoon placenta in all their large number of fetuses studied. They
were critical of Watson (1881) as having misinterpreted the remains of the
"haemophagous organ" as an interruption of the placental "annulus"
(Creed & Biggers, 1963). It is noteworthy, however, that their figure
clearly shows that the haemophagous zone interrupts the labyrinthine annulus,
to obtain its maternal blood from the basal (anti-mesometrial) endometrium.
The epitrichium has been described in camels and sloths (see those chapters)
and perhaps in the lesser panda. But there is, however, disagreement of
the nature of this "membrane" (see Biggers & Creed, 1962,
and Creed & Biggers, 1963). These authors believed that Watson (1881)
mistook the amnionic membrane as epitrichium and felt that the latter is
not a true structure. Watson described the umbilical cord as "hardly
existing", as its two arteries and one vein immediately diverged over
the placenta. He also noted the absence of the green border zone found in
other carnivora placentas. On the other hand, Biggers & Creed (1962)
specifically described the "haemophagous organ" as a separate
and different type of placentation in the raccoon. They reported it to be
present in young gestations and as nearly completely disappearing (remains
are a plaque) toward term. As to the papillary structure described of the
disk in the fixed specimen, Watson highlighted the enormous ("colossal")
nature of the fetal capillaries.
Biggers & Creed (1962) depicted in a preliminary report the identification
of the haemophagous organ in the raccoon placenta and defended this term
against the connotation of "hematoma", the latter being pathological
as they suggested. It is a sac-like structure that emanates from the center
of the placenta and "hangs into the allantoic cavity" antimesometrially.
Importantly, it is not like the marginal hematomas seen in dogs. This haemophagous
organ, as they named it, results from the infiltration/erosion of maternal
vessels by cytotrophoblast that there exists in many lamellae and is tall,
cylindrical and leads to maternal hemorrhages. The trophoblast is described
as containing colored crystals that they interpreted as being hemoglobin
breakdown products. While this structure is considered to be a type of hemochorial
placentation and is quite large early in gestation, it atrophies later and
becomes a small plaque. The same structure is found in several other carnivora,
mostly mustelidae as described by Biggers & Creed (1964). While these
authors ascribed a resorptive function to this "Blutbeutel", they
never stained it for iron, although hemoglobin breakdown is mentioned, as
is red cell phagocytosis. As will be seen below, iron stains are negative,
as is the case for other carnivores and ungulates that have subchorionic
pigment deposits.
Creed & Biggers (1963) described the labyrinth as a trophoblast-covered
lamellar structure that surround (enclose) maternal capillaries. The maternal
"capillaries" are really sinusoidal in nature and whose endothelium
is cuboidal. Then follows a thick PAS+ basal membrane. The trophoblast is
syncytial with irregularly disposed nuclei. While this type of placental
membrane can initially be described as being vaso-chorial, there is progressive
thinning so that ultimately, a hemo-endothelial separation evolves.
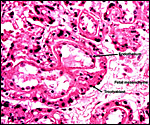
The Coatimundi also has a zonary placenta with central attachment of the short umbilical cord. The microscopic structure of the exchange organ is labyrinthine. The interface is primarily endothelio-chorial. Mossman (1987) also makes reference to large central hematomas. While the placenta I observed is bets described as having been "oval", it had originally doubtless been zonary and the yellow discoloration seen at one end is very much most likely the remains of the former haemophagous organ. It conforms in all respects to that described for the remnants of the haemophagous organ of the raccoon. Moreover, the histologic appearance is identical. Regrettably, it was detached from the uterus and the endometrium could thus not be observed.
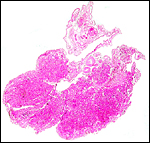 |
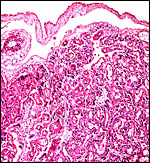
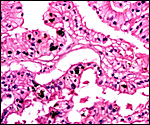
Cross section through this zonary placenta with fetal surface blood vessels above. |
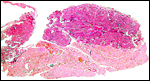 |
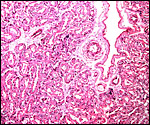
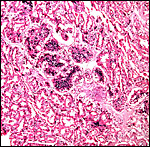
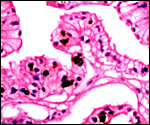
Another section with paler but histologically similar region below. |
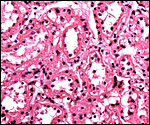
The feto-maternal barrier of Nasua is essentially the same as that of Procyon. In early gestation it would appear that the region of the haemophagous organ has a hemochorial relationship. The labyrinth is identical to that of the raccoon. There is a relatively small amount of villous connective tissue that is covered by trophoblast which, in many areas has a syncytial quality. Other regions are more cuboidal, and projections of fetal capillaries into the trophoblast are focally evident. The presenting maternal surface is largely the cuboidal, and prominent, endothelium of its vascular channels. I have not seen a closer contact between mother and fetus in the sections I possess. Beneath the chorion, the trophoblast is much more cylindrical as will be seen in the photographs below. In the central areas (towards the maternal surface) foci of concentrations of syncytiotrophoblast are present without a clear relationship to exchange function.
The umbilical cord of this specimen was 7 cm long, much longer than the length given by Starck (1957) which was given as 0.5 cm. It had no spirals and possessed three blood vessels. The surface was smooth and had no areas of squamous metaplasia. Watson (1881) described a very much shorter umbilical cord to exist in the raccoon gestation but no other information is available at this time. There have been no considerations of the length or type of cords in the many placental specimens described by Biggers and Creed.
7)
Uteroplacental circulation
Mossman (1987) described the uterus of raccoons (close relatives) as being
bicornuate with a small body, and Watson (1881) provided the only detailed
description of a pregnant uterus and its nonpregnant horn. He found a
septum between the two horns and stated that the uterine mucosa imperceptibly
changes to become vaginal tissues. He also described the uterine vasculature.
8)
Extraplacental membranes
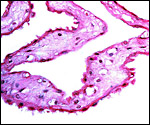
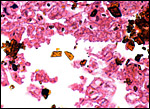
A large allantoic sac surrounds the amnionic sac. Into this allantoic
sac expands the "haemophagous organ" described earlier that
largely disappears toward term in the raccoon. I assume that a similar
situation pertains in coatis, although early stages have not been described.
There is, however, a remaining yellow portion at the expected site in
the placenta I was able to observe and this contains a large number of
yellow crystals. The wall is covered with cylindrical epithelial cells
(presumably trophoblast) that engage in phagocytosis and contain the same
crystals, as will be seen below. Blood was not found in this term placenta
of the coati. Despite the fact that this haemophagous organ is speculated
to supply iron to the fetus from the maternal hematoma, no iron staining
(Prussian blue stains) occurred. No hippomanes are present.
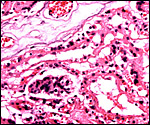
No endometrium was available, but from what is known of raccoon placentation, trophoblast invasion does not occur.
10)
Endometrium
The placenta of raccoons and coatis is described as being deciduate, but
lacking the uterus of this specimen, the endometrium cannot be described
and it has not been reported. There is no decidua found attached to the
delivered placenta, only a small amount of fibrin is present. Focal calcifications
are present in the connective tissue radiating from the maternal floor
into the labyrinth.
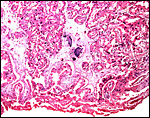 |
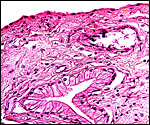
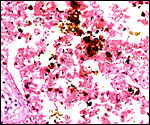
This section is from the placental floor and shows a focus of calcification (purple) in decidual remnants. |
No special features are known to me.
12) Endocrinology
Watson (1881) described the ovary of the raccoon as being lobulated and encircled by the Fallopian tube but lacking a bursa. This is contrary to the observation of Mossman & Duke (1973) who described a complete bursa.
13) Genetics
Coatimundis have 38 chromosomes as shown below (from, Hsu & Benirschke, 1970). A variety of other studies cited there have confirmed this karyotype. Wurster-Hill & Gray (1975) have studied other procyonids (all 2n=38) with Giemsa banding and found structural differences among them but they also remarked at the great similarity of some chromosomes to those of felidae. Hybrids have not been described. Zhang & Ryder (1993) examined the mitochondrial DNA sequence evolution of many carnivora and found that lesser pandas were distinct from procyonids and that, as a group, the procyonids separated from each other early in their evolution. Both species (N. narica and N. nasua) have the same chromosomes (see Hsu & Arrighi, 1966; Todd et al., 1966; Panzetta & Alaimo, 1967; Wurster & Benirschke, 1968). A “marker chromosome” with secondary constriction is present in all and is very similar in structure. In general, the karyotype is not very different from that of Felidae; but serologically these families are not closely related as relevant studies showed in the past (Leone & Wiens, 1956; Pauly & Wolfe, 1957).
 |
Karyotypes of Nasua narica. |
 |
Karyotypes of Nasua narica. |
Costa et al. (1995) studied the delayed hypersensitivity reaction to paracoccidioidin in captive arboreal vs. terrestrial animals of Brazil ; they found the former to have a much lower reactivity (22 vs. 83%).
15)
Pathological features
Reppas et al. (2001) described two coatimundis with what were interpreted
to be bilateral pheochromocytomas. Infectious diseases are important problems
for coatis. Thus, Lainson & Shaw (197905) reported fatal pneumocystis
infection and, later (1979), they identified organisms similar to Trypanosoma
cruzi (the cause of Chagas' disease) in coatis and considered this
as a possible health hazard for humans. Trypanosoma evansi, the
cause of an important equine disease in Brazil, was isolated from coatis
by Quiroz et al. (2000) An experimental study of infecting coatis with
this agent led to severe anemia, myocarditis and encephalitis (Herrera
et al., 2002). Griner (1983) found tuberculosis (Mycobacterium tuberculosis)
in two animals at the San Diego Zoo. This led to the euthanasia of the
remainder of animals in the colony which disclosed only dermatitis and
mite infection.
Numerous infectious and parasitic afflictions have been described for this species. Thus, Schmidt (1977) identified Onicola luehei in animals from Paraguay; Trypanosoma cruzi-like organisms were identified by Lainson et al. (1979) in Brazilian animals; Trypanosoma evansi was isolated from coatis by Quieroz et al. (2000) and the course of infection followed after experimental transmission (Herrera et al. (2002); Leptospira were isolated from kidneys of Nasua by Lins & Lopes (1984); Toxocara cancrivorus was found by Sprent (1982); Valenzuela et al. (2000) described an epizootic of mange (due to Notoedres cati) in Mexico; Salgado-Maldonado & Cruz-Reyes (2002) found the acantocephalan Porrorchis nickoli also in Mexican animals; fatal infection with Pneumocystis carinii in a freshly-caught coati from the Amazonas; Baird & Neafie (1988) refer to infection with Brugia guyanensis a nematode from South America; over 80% of Brazilian coatis tested positive in an epidemiological study of histoplasmosis, and 65% for antigens of sporotrichosis; Leishmania shawi was found in coatis from Brazil by Lainson et al. (1989) who subsequently studied the possible origin of this infection in patients (Shaw et al., 1991); the animal described here had died from cryptosporidiosis, and Jatobal virus was isolated from a coati in Brazil.
Chittick et al. (2001) described pyometra and an endometrial carcinoma in a coati that had been implanted with melengestrol acetate for 4.5 years.
16) Physiologic data
Boggs & Irvine (1992) studied the respiratory mechanics of coatimundis
and found them to be significantly different from the breathing patterns
of woodchucks in that the coati had a larger tidal volume. Grant et al.
(1976) had earlier made systematic studies of the regulation of pulmonary
blood flow. Brimhall et al. (1979) as well as Ahmed et al. (1990) studied
hemoglobin composition of coatis, mink and hyenas, while Urashima et al.
(1999) examined the milk saccharides of coatis and identified two novel
pentasaccharides. McClearn (1985) studied the fiber length of skeletal
muscle fibers of distal arm and leg muscles in coatis and compared them
with those of the raccoon. The coati is primarily diurnal, while the activity
of kinkajou and raccoon occur mainly at night. For that reason, Jacobs
& Deegan (1992) studied their retinal pigment and found that the nocturnal
species were monochromatic, while the coati was dichromatic.
17)
Other resources
Cell strains of a number of Coatimundis can be made available from CRES
at San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
There have been no descriptions of early stages of coati placentation;
they are badly needed. Likewise, a better description of allantois and
length of umbilical cord are needed. There are no endocrine studies, despite
the relative wide distribution of the species in the Americas and in zoos.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society
of San Diego
References
Ahmed, A., Jahan, M. and Braunitzer, G.: Carnivora: the primary structure
of hemoglobin from adult coati (Nasua nasua rufa, Procyonidae).
J. Protein Chem. 9:23-29, 1990.
Baird, J.K. and Neafie, R.C.: South American brugian filariasis: report of a human infection acquired in Peru . Amer. J. Trop. Med. Hyg. 39:185-188, 1988.
Beisiegel, B.M.: Notes on the coati, Nasua nasua (Carnivora: Procyonidae) in an Atlantic forest area. Braz. J. Biol. 61:689-692, 2001.
Biggers, J.D. and Creed, R.F.S.: Two morphological types of placentae in the raccoon. Nature 194:103-105, 1962.
Boggs, D.F. and Irvin, C.G.: Respiratory mechanics of the coatimundi and woodchuck. Respir. Physiol. 89:147-155, 1992.
Brimhall, B., Stenzel, P., Dresler, S.L., Hermodson, M., Stangland, K., Joyce, J. and Jones, R.T.: On the tryptic peptides from hemoglobin chains of six carnivores. J. Mol. Evol. 9:237-260, 1977.
Brimhall, B., Stangland, K., Jones, R.T., Becker, R.R. and Bailey, T.J.: Tryptic peptide composition of hemoglobins from mink (Mustela vison) and hyena (Hyaenae hyaenae) and the alpha-chain of coatimundi (Nasua nasua). Hemoglobin 3:271-292, 1979.
Chittick, E., Rotstein, D., Brown, T. and Wolfe, B.: Pyometra and uterine adenocarcinoma in a megestrol acetate-implanted captive coati (Nasua nasua). J. Zoo Wild. Med. 32:245-251, 2001.
Costa, E.O., Diniz, L.S., Netto, C.F., Arruda, C. and Dagli, M.L.: Epidemiological study of sprorotrichosis and histoplasmosis in captive Latin American wild mammals, Sao Paulo , Brazil . Mycopathologia 125:19-22, 1994.
Costa, E.O., Diniz, L.S., Netto, C.F., Arruda, C. and Dagli, M.L.: Delayed hypersensitivity test with paracoccidioidin in captive Latin American wild mammals. J. Med. Vet. Mycol. 33:39-42, 1995.
Creed, R.F.S. and Biggers, J.D.: Development of the raccoon placenta. Amer. J. Anat. 113:417-445, 1963.
Creed,
R.F.S. and Biggers J.D.: Placental haemophagous organs in the procyonidae
and mustelidae. J. Reprod. Fertil. 8:133-137, 1964.
Gompper , M.E. , Gittleman, J.L. and Wayne , R.K.: Dispersal, philopatry, and genetic relatedness in a social carnivore: comparing males and females. Mol. Evol. 7:157-1634, 1998.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Grant, B.J., Davies, E.E., Jones, H.A. and Hughes, J.M.: Local regulation of pulmonary blood flow and ventilation ratios in the coatimundi. J. Appl. Physiol. 40:216-228, 1976.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego,
California, 1983.
Herrera, H.M., Alessi, A.C., Marques, L.C., Santana, A.E., Aquino, L.P.,
Menezes, B.F., Moraes, M.A. and Machado, R.Z.: Experimental Trypanosome
evansi infection in South American coati (Nasua nasua): hematological,
biochemical and histopathological changes. Acta Trop. 81:203-210, 2002.
Hsu, T.C. and Arrighi, F.E.: Karyotypes of 13 carnivores. Mammal. Chromosom. Newsl. 21:155, 1966.
Hsu, T.C. and Benirschke, K.: A Mammalian Chromosome Atlas. Volume 4, Folio 183, 1970. Springer-Verlag, N.Y.
Jacobs, G.H. and Deegan, JF 2nd.: Cone photopigments in nocturnal and diurnal procyonids. J. Comp. Physiol. (A) 171:351-358, 1992.
Jones, M.L.: Longevity of captive mammals. Zool. Garten 52:113-128, 1982.
Lainson, R. and Shaw, J.J.: Pneumocystis and histoplasma infections in wild animals from the Amazon region of Brazil. Trans. Roy. Soc. Trop. Med. Hyg. 69:505-508, 1975.
Lainson,
R., Shaw, J.J., Fraiha, H., Miles, M.A. and Draper, C.C.: Chagas' disease
in the Amazon Basin: 1. Trypanosoma cruzi infections in silvatic
mammals, triatome bugs and man in the State of Para, north Brazil. Trans.
Roy. Soc. Trop. Med. Hyg. 73:193-204, 1979.
Lainson, R., Braga , R.R., de Souza, A.A., Povoa, M.M., Ishikawa, E.A. and Silveira, F.T.: Leishmania (Viannia) shawi sp. N., a parasite of monkeys, sloths and procyonids in Amazonia Brazil . Ann. Parasitol. Hum. Comp. 64:200-207, 1989.
Leone , C.A. and Wiens, A.L.: Comparative serology of carnivores. J. Mammal. 37:11-23, 1956.
Lins, Z.C. and Lopes, M.L.: Isolation of Leptospira from wild forest animals in Amazonian Brazil. Trans. R. Soc. Trop. Med. Hyg. 78:124-126, 1984.
McClearn, D.: Anatomy of raccoon (Procyon lotor) and coati (Nasua narica and N. nasua) forearm and leg muscles: relations between fiber length, moment-arm length, and joint-angle excursion. J. Morphol. 183:87-115, 1985.)
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mossman, H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University of Wisconsin Press, Madison, Wisconsin, 1973.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Panzetta, P. and Alaimo, I. : Karyotype of the coati (Coati nasua solitaria Schinz). Mammal. Chromosom. Newsl. 8:97, 1967.
Pauly, L.K. and Wolfe, H.R.: Serological relationships among members of the order Carnivora. Zoologica 42:159-166, 1957.
Quieroz, A.O., Nehme-Russell, N.S., Brandao, A. and Jansen, A.M.: Homogeneity of Trypanosoma evansi isolates from domestic and sylvatic mammals from the Pantanal of Mato Grosso. Microbios 103:27-30, 2000.
Reppas,
G.P., Bodley, K.B., Watson, G.F. and Wills, E.J.: Phaeochromocytoma in
two coatimundis (Nasua nasua) Vet. Rec. 148:806-809, 2001.
Saeed, M.F., Wang, H., Suderman, M., Beasley, D.W., Travassos da Rosa, A., Li, L., Shope, R.E., Tesh, R.B. and Barrett, A.D.: Jatobal virus is a reassortant containing the small RNA of Oropouche virus. Virus Res. 77:25-230, 2001.
Salgado-Maldonado, G. and Cruz-Reyes, A.: Porrorchis nickoli n. sp. (Acanthocephala: Plagiorhynchidae) from mammals in southeastern Mexico , first known occurrence of Porrorchis in the western hemisphere. J. Parasitol. 88:146-152, 2002.
Schmidt, G.D.: Onicola martini sp. n., and other archiacanthocephala of the Chaco Boreal, Paraguay . J. Parasitol. 63:508-510, 1977.
Shaw, J.J., Ishikawa, E.A., Lainson, R., Braga, R.R. and Silveira, F.T.: Cutaneous leishmaniasis of man due to Leishmania (Vianna) shawi Lainson, de Souza, Povoa, Ishikawa & Silveira, in Para State, Brazil. Ann. Parasitol. Hum Comp. 66:243-246, 1991.
Sprent, J.F.: Ascaridoid nematodes of South American mammals, with a definition of a new genus. J. Helminthol. 56:275-295, 1982.
Starck, D.: Ueber die Länge der Nabelschnur bei Säugetieren. Z. Säugetierk. 22:77-86, 1957.
Timock, J. and Vaughan, C.: A census of mammal populations in Punta Leona Private Wildlife Refuge, Costa Rica . Rev. Biol. Trop. 50:1169-1180, 2002.
Todd, N.B., York , R.N. and Pressman, S.R.: The karyotypes of the raccoon (Procyon lotor L.), Coatimundi (Nasua narica L.) and kinkajou (Potos flavus Schreber). Mammal. Chromosom. Newsl. 8:97, 1966.
Urishima,
T., Yamamoto, M., Nakamura, T., Arai, I., Saito, T., Namiki, M., Yamaoka,
K. and Kawahara, K.: Chemical characterization of the oligosaccharides
in a sample of milk of a white-nosed coati, Nasua narica (Procyonidae,
Carnivora). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 123:187-193,
1999.
Vacek, Z.: Placenta of Nasua rufa L. Biol. Listy 31 (suppl.):155-163, 1951.
Valenzuela, D., Ceballos, G. and Garcia, A.: Mange epizootic in white-nosed coatis in western Mexico . J. Wildl. Dis. 36:56-63, 2000.
Watson, M.: On the female organs and placentation of the raccoon (Procyon lotor). Proc. Roy. Soc. London 32:272-298, 1881.
Wilson,
G.G.: Splendid Isolation. The curious History of South American Mammals.
Yale University Press, New Haven, 1980.
Wurster, D.H. and Benirschke, K.: Comparative Cytogenetic studies in the order Carnivora. Chromosoma 24:336-382, 1968.
Wurster-Hill, D.H. and Gray, C.W.: The interrelationships of chromosome banding patterns in procyonids, viverrids, and felids. Cytogenet. Cell Genet. 15:306-331, 1975.
Zhang, Y.P. and Ryder, O.A.: Mitochondrial DNA sequence evolution in the Arctoidea. Proc. Nat. Acad. Sci. USA 90:9557-9561, 1993.