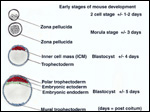
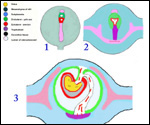
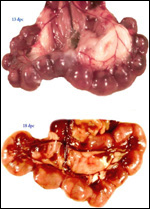
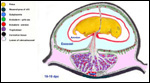
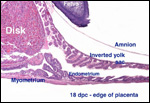
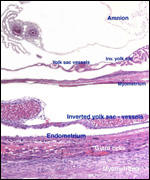
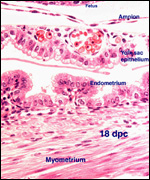
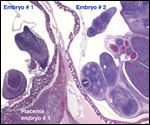
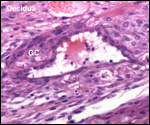
| |
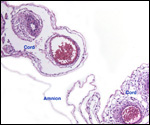
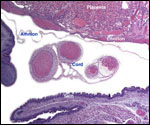
Monozygotic
twins with shared yolk sacs and shared circulation were described by Runner
(1984). A plethora of diseases of mice and congenital anomalies have been
reported, but there are too many studies to be covered in this context.
The reader is referred to two comprehensive reviews (Benirschke et al.,
1978; Knobil & Neill, 1998).
16)
Physiological data
I have no data or references pertaining to the magnitude of the maternal
blood flow, blood volume, or blood pressure of mice.
17)
Other resources
Cell lines are available from the Bar Harbor Laboratories and many other
agencies. The mouse has been an extraordinary model to study certain genes
by the creation of "knockout" mice. There are so many models
now available that the topic has become difficult to oversee. For that
reason, a "Mouse Knockout & Mutation Database" (MKMD) has
been developed in 1995 that now lists over 7000 entries. A sample can
be viewed at http://research.bmn.com/mkmd. Ward et al. (2000) published
a book on the pathology of genetically engineered mice that may be helpful.
18)
Other relevant features
Embryo transfer of mice has been possible since 1935 and is reviewed by
Kraemer (1983). This publication also provides references and technical
details that may be helpful to the experimenter. Intraspecific chimerae
have been produced several times, but the rat x mouse aggregation of blastomeres,
while leading to normal appearing blastocysts, did not yield grown chimeric
embryos (Stern, 1973). Surani & Barton (1983) produced gynecogenetic
embryos, but they failed to go beyond the 25-somite stage. This is presumably
due to homozygosity for lethal genes, similar to the androgenetic hydatidiform
moles in humans which are lethal to the embryo.
19) Future research needs
There is much information to be gained from the study of mouse placentation.
This is especially true for our understanding of the genetic regulation
of placentation and trophoblast regulation. The publication by Georgiades
et al. (2002) should be consulted for references and directions. In view
of the numerous mouse mutations produced, it will be interesting to learn
more about the possibly accompanying placental lesions.
References
Anson-Cartwright, L., Dawson, K., Holmyard, D., Fisher, S.J., Lazzarini,
R.A. and Cross, J.C.: The glial cells missing-1 protein is essential for
branching morphogenesis in the chorioallantoic placenta. Nature Genetics
25:311-314, 2000.
Barlow,
P.W. and Sherman, M.I.: The biochemistry of differentiation of mouse trophoblast:
Studies on polyploidy. J. Embryol. Exp. Morphol. 27:447-465, 1972.
Barlow,
P.W. and Sherman, M.I.: Cytological studies on the organization of DNA
in giant trophoblastic nuclei of the mouse and the rat. Chromosoma 47:119-131,
1974.
Benirschke,
K.: Spontaneous chimerism in mammals. A critical review. Current Topics
in Pathology 51:1-61, 1970. (Springer-Verlag, NY).
Benirschke,
K., Garner, F.M. and Jones, T.C.: Pathology of Laboratory Animals. Springer-Verlag,
NY 1978.
Birken,
S., O'Connor, J., Lobel, L. and Canfield, R.: Chorionic Gonadotropin,
Human. Pp. 587-614, in Volume 1 of: Encyclopedia of Reproduction , E.
Knobil and J.D. Neill eds.: Academic Press, San Diego, 1998.
Brambell,
F.W.R.: The transmission of immunity from mother to young and the catabolism
of immunoglobulins. Lancet 2:1087-1093, 1966.
Carpenter,
S.J. and Ferm, V.H.: Uptake and storage of thorotrast by the rodent yolk
sac placenta: An electronmicroscopic study. Amer. J. Anat. 125:429-456,
1969.
Castiglia,
R. and Capanna, E.: Whole-arm translocation (WART) in a feral population
of mice. Chromosome Res. 7:493-495, 1999.
Cohen,
J. and Feldberg, D.: Effects of the size and number of zona pellucida
openings on hatching and trophoblast outgrowth in the mouse embryo. Mol.
Reprod. Dev. 30:70-78, 1991.
Committee
on standardized genetic nomenclature for mice: Standard karyotype of the
mouse. J. Hered. 63:69-72, 1972.
Copp,
A.J.: Interaction between inner cell mass and trophectoderm of the mouse
blastocyst. I. A study of cellular proliferation. J. Embryol. Exp. Morphol.
48:109-125, 1978.
Copp,
A.J.: The development of field vole (Microtus agrestis) and mouse
blastocysts in vitro: A study of trophoblast cell migration. Placenta
1:47-60, 1980.
Cross, J.C.: The mouse as a model for investigation of placental function.
Chapter 5 (pp.51-60) in, The Placenta: Basic Science and Clinical Practice.
J. Kingdom, E. Jauniaux and S. O'Brien, eds. Royal College of Obstetricians
and Gynaecologists (RCOG) Press, London, 2000a.
Cross,
J.C.: Genetic insights into trophoblast differentiation and placental
morphogenesis. Seminars Cell Developm. Biol. 11:105-112, 2000b.
Cross,
J.C., Lu, Y., Hemberger, M. and Adamson, L.: Invasion of the mouse uterus
by distinct trophoblast subtypes. Placenta 22 (7): abstract P64, p. A24,
2001.
Cross, J.C., Werb, Z. and Fisher, S.J.: Implantation and the placenta:
Key pieces of the development puzzle. Science 266:1508-1518, 1994.
Croy,
B.A., Rossant, J. and Clark, D.A.: Histological and immunological studies
of post implantation death of Mus caroli embryos in the Mus
musculus uterus. J. Reprod. Immunol. 4:277-293, 1982.
Dickson,
A.D.: Trophoblastic giant cell transformation of mouse blastocysts. J.
Reprod. Fertil. 6:465-466, 1963.
Dickson,
A.D.: Variations in development of mouse blastocysts. J. Anat. 101:263-267,
1967.
Douarin,
N. le and McLaren, A.: Chimeras in Developmental Biology. Academic Press,
London, 1984.
Downs, K.M.: Early placental ontogeny in the mouse. Placenta 23:116-131,
2002.
Finn,
C.A. and Lawn, A.M.: Specialized junctions between decidual cells in the
uterus of the pregnant mouse. J. Ultrastr. Res. 20:321-327, 1967.
Gardner,
R.L. and Johnson, M.H.: An investigation of inner cell mass and trophoblast
tissues following their isolation from the mouse blastocyst. J. Embryol.
Exp. Morphol. 28:279-312, 1972.
Georgiades,
P., Watkins, M., Burton, G.J. and Ferguson-Smith, A.C.: Roles for genomic
imprinting and the zygotic genome in placental development. Proc. Natl.
Acad. Sci. USA 98:4522-4527, 2001.
Georgiades, P., Ferguson-Smith, A.C. and Burton, G.J.: Comparative developmental
anatomy of the murine and human definitive placentae. Placenta 23:3-19,
2002.
Gill,
T.J., Siew, S. and Kunz, H.W.: Major histocompatibility complex (MHC)-linked
genes affecting development. J. Exp. Zool. 228:325-345, 1983.
Giudice,
L.C.: Genes associated with embryonic attachment and implantation and
the role of progesterone. J. Reprod. Med. 44:165-171, 1999 (Suppl. 2).
Gray,
A.P.: Mammalian Hybrids. Second edition. A Check-List with Bibliography.
Commonwealth Agricultural Bureaux, Farnham Royal, Slough, UK, 1972.
Gropp,
A., Winking, H., Redi, C.A., Capanna, E., Britton-Davidian, J. and Noack,
G.: Robertsonian karyotype variation in wild house mice from Rhaeto-Lombardia.
Cytogenet. Cell Genet. 34:67-77, 1982.
Gropp,
A., Winking, H., Herbst, E.W. and Claussen, C.P.: Murine trisomy: Developmental
profiles of the embryo, and isolation of trisomic cellular systems. J.
Exp. Zool. 228:253-269, 1983.
Heine,
H.: Histoautoradiographie der DNS in Trophoblastriesenzellen der Plazenta
der Maus. Acta anat. 95:66-74, 1976.
Heinecke,
H. and Grimm, H: Plazentafusionen bei verschiedenen Mausestammen. Z. Versuchstierk.
13:224-231, 1971.
Hemberger,
M. and Cross, J.C.: Genes governing placental development. Trends Endocrinol.
Metab. 12:162-168, 2001.
Ito,
K., McGhee, J.D. and Schultz, G.A.: Paternal DNA strands segregate to
both trophectoderm and inner cell mass of the developing mouse embryo.
Genes & Development 2:929-936, 1988.
Iwaki, T., Sandoval-Cooper, M.J., Paiva, M., Kobayashi, T., Ploplis, V.A.
and Castellino, F.J.: Fibrinogen stabilizes placental-maternal attachment
during embryonic development in the mouse. Amer. J. Pathol. 160:1021-1034,
2002.
Kaufman,
M.H.: Two examples of monoamniotic monozygotic twinning in parthenogenetic
mouse embryos. J. Exp. Zool. 224:277-282, 1982.
Kaufman,
M.H.: The Atlas of Mouse Development. Academic Press, 1992.
Kirby,
D.R.S. and Bradbury, S.: The hemochorial mouse placenta. Anat. Rec. 152:279-282,
1965.
Knobil,
E. and Neill, J.D.: Encyclopedia of Reproduction. Academic Press, San
Diego, 1998.
Koren,
Z., Damme, O.v., Hirumi, H. and Maramorosch, K.: Growth and stimulation
of mouse trophoblastic cells in culture by polyoma virus. Amer. J. Obstetr.
Gynecol. 111:846-850, 1971.
Kraemer,
D.C.: Intra- and interspecific embryo transfer. J. Exp. Zool. 228:363-371,
1983.
Kunath,
T., Strumpf, D., Rossant, J. and Tanaka, S.: Trophoblast stem cells. Chapter
12 (pp. 267-287) in, Stem Cell Biology. D.R. Marshak, R.L. Gardner and
D. Gottlieb, eds. Cold Spring Harbor Laboratory Press, Cold Spring Harbor,
NY, 2001 (Monograph 40).
Li,
Y. and Behringer, R.R.: Essx1 is an X-chromosome-imprinted regulator of
placental development and fetal growth. Nature Genetics 20:309-309, 1998.
Kutsche, K. and Gal, A.: The mouse Arhef6gene:cDNA sequence, expression
analysis, and chromosome assignment. Cytogenet. Cell Genet. 95:196-201,
2001.
Luo,
J., Sladek, R., Bader, J.-A., Matthyssen, A., Rossant, J. and Giguère,
V.: Placental abnormalities in mouse embryos lacking the orphan nuclear
receptor ERR-ß. Nature 388:778-782, 1997.
McLaren,
A., Molland, P. and Signer, E.: Does monozygotic twinning occur in mice?
Genet. Res. 66:195-202, 1995.
Mossman,
H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nesbitt,
M.N.: Evolutionary relationships between rat and mouse chromosomes. Chromosoma
46:217-224, 1974.
Philipp, M., Brede, M.E., Hadamek, K., Gessler, M., Lohse, M.J. and Hein,
L.: Placental a2-adrenoceptors control vascular development at the interface
between mother and embryo. Nature Genetics 31:311-315, 2002.
Ramsey,
E.M.: The Placenta of Laboratory Animals and Man. Holt, Rinehart and Winston,
New York, 1975.
Rossant, J. and Cross, J.C.: Placental development: Lessons from mouse
mutants. Nature Reviews Genetics 2:538-548, 2001.
Rossant,
J., Croy, B.A., Clark, D.A. and Chapman, V.M.: Interspecific hybrids and
chimeras in mice. J. Exp. Zool. 228:223-233, 1983.
Rossant,
J. and Croy, B.A.: Genetic identification of tissue of origin of cellular
population within the mouse placenta. J. Embryol. Exp. Morph. 86:177-189,
1985.
Rossant,
J. and Cross, J.C.: Placental development: Lessons from mouse mutants.
Nature Reviews Genetics 2:538-548, 2001.
Runner,
M.N.: New evidence for monozygotic twins in the mouse: Twinning initiated
in the late blastocyst can account for mirror images asymmetries. Anat.
Rec. 209:399-406, 1984.
Shaver,
E.L. and Martin-DeLeon, P.A.: Effects of aging of sperm in the female
and male reproductive tracts before fertilization on the chromosome complement
of the blastocysts. In, Aging Gametes. R.J. Blandau, ed. Pp. 151-165.
Karger, Basel, 1975.
Snow,
M.H.L. and Ansell, J.D.: The chromosomes of giant trophoblast cells of
the mouse. Proc. R. Soc. Lond. B.: 187:93-98, 1974.
Sotomaru,
Y., Kato, Y. and Tsunoda, Y.: Production of monozygotic twins after freezing
and thawing of bisected embryos. Cryobiology 37:139-145, 1998.
Stern,
M.S.: Chimaeras obtained by aggregation of mouse eggs with rat eggs. Nature
243:472-473, 1973.
Sun,
F.L., Dean, W.L., Kelsey, G., Allen, N.D. and Reik, W.: Transactivation
of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature
389:809-815, 1997.
Surani,
M.A.H. and Barton, S.C.: Development of gynecogenetic eggs in the mouse:
Implications for parthenogenetic embryos. Science 222:1034-1036, 1983.
Takagi,
N. and Sasaki, M.: Preferential inactivation of the paternally derived
X-chromosome in the extraembryonic membranes of the mouse. Nature 256:640,
1975.
Tarkowski,
A.K., Ozdenski, W. and Czolowska, R.: Mouse singletons and twins developed
from isolated diploid blastomeres supported with tetraploid blastomeres.
Int. J. Dev. Biol. 45:591-596, 2001.
Theiler,
K.: The House Mouse. Development and normal stages from fertilization
to 4 weeks of age. Springer-Verlag, NY 1972.
Ward, J.M., Mahler, J., Maronpot, R.R., Sundberg, J.P. and Frederickson,
R. (eds): Pathology of Genetically Engineered Mice. Iowa State University
Press, Ames, 2000.
Ward, J.M. and Devor-Henneman, D.E.: Gestational mortality in genetically
engineered mice: Evaluating the extraembryonal embryonic placenta and
membranes. Pp. 103-122, in: Ward, J.M., Mahler, J., Maronpot, R.R., Sundberg,
J.P. and Frederickson, R. (eds): Pathology of Genetically Engineered Mice.
Iowa State University Press, Ames, 2000.
Wallace,
M.E. and Williams, D.A.: Monozygotic twinning in mice. J. Med. Genet.
2:26-31, 1965.
West,
J.D., Freis, W.I. and Chapman, V.M.: Mus musculus x Mus caroli
hybrids: mouse mules. J. Hered. 69:321-326, 1978.
Yuan, L., Liu, J.-G., Hoja, M.-R., Wilbertz, J., Nordqvist, K. and Höög,
C.: Female germ cell aneuploidy and embryonic death in mice lacking the
meiosis-specific protein SCP3. Science 296:1115-1118, 2002.
|