|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
| Last updated: October 26, 2004. |
Gazella dama mhorr
Order: Artiodactyla
Family: Bovidae (Subgenus Nanger)
1) General Zoological Data
Historically, this species was once distributed from Southern Morocco to Senegal, but now it is a very much endangered species. In Africa, the species has been eradicated from its last habitat, the largest and western-most distribution of the Dama gazelle or "Addra" gazelle in Morocco (Dolan, 1981). A small group of gazelles from the Southern Spanish Sahara was removed to Spain in 1971, which saved the species. It comprised 2.12 animals (not counting the sterile animals), all captive-bred. The species now exists in several breeding colonies in zoological gardens after groups of these gazelles were obtained by various zoological parks from Almeira, Spain. The original founder population is generally given as n=12 and some 240 animals currently live in zoos (Wiesner & Muller, 1998). A significant breeding group also exists at the San Diego Zoo. It began with 1.3 Mhorr gazelles that were imported from Spain in 1981. Wiesner & Muller (1998) reviewed the effectiveness of the reintroduction of some animals to Tunisia and Morocco in 1990-1992.
This rare animal was known in the past as producing precious bezoars, the "Baid-al-Mhorr" (eggs of M'horr) according to Dolan's description (1981) and thus received its name. The animal is often also called the Mohor gazelle.
Drüwa (1985) has provided us with an extensive review of all Dama gazelles in zoos, their feed, reproduction, longevity and causes of death. Adults may live to 17 years of age, singleton births are the rule and he was especially keen on emphasizing the susceptibility of these animals to colds. Grettenberger & Newby described the habitat and biology of Dama gazelles in Niger. Jones (1993) gives as longevity for Mhorr gazelles an age of 14 years 10 months.
Lange (1971) studied a large number of skulls of various "Nanger gazelles" and, in considering their numerous subspecies, he also evaluated their coloration and distribution in Africa. He concluded that true Mhorr gazelles were extinct. Eigener & Schliemann (1983) suggested that the Mhorr gazelles that are now seen in zoos are actually Dama gazelles and that the true Gazella dama mhorr is extinct. They deduced that the origin of "mhorr" is from the Arabic "mhur", a designation for various young animals, not only gazelles. This darkest of all Dama gazelles is, they believe, merely a variant of Gazella dama. It is perhaps appropriate to state that, now, the Mhorr gazelle is considered to be one of the three subspecies of Dama gazelle (G. dama dama, G. d. mhorr, G. d. ruficollis). One characteristic of the "Nanger" gazelles is that, from West to East of their distribution, the dark red-brown coloration of their back decreases in extent and that it becomes lighter in color further East. These color variations were considered most intensively by Perez (1984) who also concluded that three subspecies of Dama gazelles can be differentiated according to their coloration. She correlated these colorations with known distributions in the past. The correlation with karyotypic findings is discussed under the section of genetics below.
 |
Three Mhorr Gazelles at San Diego Zoo. |
 |
Breeding colony of Mhorr gazelles at San Diego Zoo. The dark coloration of forelegs is a characteristic of Mhorr gazelles. |
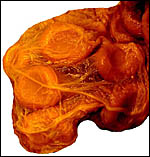
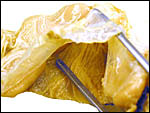
Singletons are born following pregnancy that is said to be approximately 200 days long. This gestational length is supported by several figures given in the large summary of Drüwa (1985). I am not aware of twins having been recorded. Artificial insemination has recently been successful (v.i.). The only immature placenta available to me comes from a 7 ½ year-old female whose leg fractured in November, 1991. Its uterus and fetus are depicted below. A term, delivered placenta of a 1982 birth weighed 300 g and had close to 70 cotyledons measuring 2.5 cm in average diameter. In the lesser horn the cotyledons were somewhat smaller (1.5 cm). ). An additional placenta became available in 2003. It weighed 247 g, had 36 cotyledons in the occupied horn and 35 very small cotyledons (in four rows) in the lesser horn. An additional specimen became available of a term, surviving infant. The placenta had 60 cotyledons, measured 65 x 35 cm, had a 7 cm umbilical cord and weighed 180 g. It is shown next.
3) Implantation
 |
Term placenta of Mhorr gazelle - 60 cotyledons. |
 |
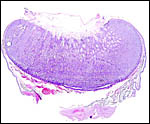
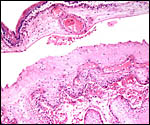
Immature Mhorr gazelle placenta implanted in bicornuate uterus, after formalin fixation. |
 |
Immature female fetus (formalin fixed) of placenta above. |
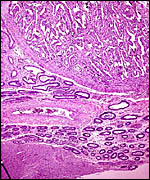
This is a polycotyledonary, partially epithelio-chorial and partially syndesmochorial placenta with "concave" cotyledons. I have had one immature placenta available from a maternal death. It had a placental implantation into both uterine horns. The immature fetus is shown above. This female fetus weighed 316 g and was 24 cm in crown-rump length. It was normally formed and had two nipples. There were approximately 60 cotyledons, each measuring 2-3 cm in diameters in diameters and 1 cm in thickness. The ovary with corpus luteum was on the right side with an adjacent large peritubal cyst.
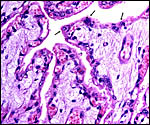
A term delivered placenta from a birth in 1982 weighed 300 g, measured 75 cm in greatest length and 20 cm in greatest diameter. It had 70 cotyledons. As indicated above, a second term placenta of a surviving neonate weighed 247 g and had 36 large cotyledons in the larger horn and 35 very small cotyledons in the unoccupied uterine horn. A placenta of Gazella rufifrons (admittedly a more distantly related taxon) was described in admirable detail by Krölling (1931); no other reports are available. He had a term uterus with the placenta attached for study and was of the opinion that the caruncular epithelium was largely destroyed and that, thus, a partially syndesmochorial placenta was formed. His placenta occupied only the left uterine horn (the right being a "mere appendage") and had 60 cotyledons. Also contrary to my observations, his placenta contained abundant yellow pigment ("hemoglobin crystals") inclusions. Otherwise, our findings are very similar and his drawings and comparison with kob and goat are exemplary. Nevertheless, he described that, contrary to observations of goat and sheep cotyledons, the gazelle cotyledons were not covered laterally by endometrium. As the photographs below show, however, I found this to be the case. In fact, the endometrium extended close to the chorionic plate on all sides.
A recent term placenta weighed 360 g, measured 69x18 cm, and had 84 cotyledons. In October 2004 yet another term placenta became available. It weighed only 120 g and had 55 cotyledons but was complete and the neonate thrived.
The single-layered trophoblast directly abuts the endometrial epithelium and connective tissue in the cotyledons. There are many regions of intact endometrial surface epithelium, especially at the tips of villi, in the caruncular crypts. Here, the epithelium significantly flattened and occasionally disrupted. Considerable debris is found at the upper portions of the endometrial tissue; some of this may be due to autolysis but it appears that the endometrial epithelium is at least partially destroyed. Thus, large areas are truly syndesmochorial. A striking dilatation of fetal capillaries (with indentation into the trophoblast) is found which, one has to assume, aids nutrient exchange. The trophoblast is single-layered and cuboidal, except beneath the chorionic plate where the single-layered trophoblast is cylindrical but, in contrast to so many other ungulates, it shows no pigmentary inclusions. Binucleate trophoblast cells are frequent on the villous surfaces. They possess the characteristically deeply-staining nuclei and dark cytoplasm. In contrast to the description given by Krölling (1931) for G. rufifrons, the endometrium in my specimen extends laterally on the cotyledons to the chorionic surface on all sides of the cotyledons. The villous stroma of the mature placenta is relatively abundant and sparsely populated by connective tissue cells. Beneath the chorionic plate are foci of villous degeneration and focal chronic villitis. Organisms were not found.
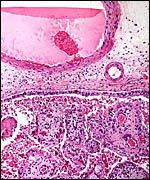
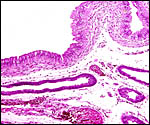
The umbilical cord of the immature fetus measured 7 cm in length and was not spiraled. It was covered by numerous small granules of squamous metaplasia. The cords of both placentas possessed two large veins and two arteries, in addition to the allantoic duct. There were a relatively small number of small blood vessels to accompany the allantoic duct, and only small numbers of smooth muscle bundles were present.
The recently obtained term placenta had a 13 cm long umbilical cord with four blood vessels and an allantoic duct.
 |
Cord of Mhorr gazelle placenta with squamous metaplasia at arrows and central allantoic duct. |
 |
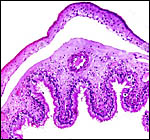
Surface squamous metaplasias of umbilical cord. |
There are no descriptions of the circulation, other than those by Krölling (1931). His drawings and my photographs above show the abundant fetal vascular supply to the cotyledons.
8)
Extraplacental membranes
The amnion is thin, translucent and avascular. Its epithelium is single-layered
and flat. Small foci of squamous metaplasia are present, particularly
near the insertion of the umbilical cord. The allantoic membrane carries
numerous fetal blood vessels and has a relatively flat epithelium. Hippomanes
were absent.
There is no invasion of trophoblast at the base of cotyledons, but the superficial endometrial cells degenerate. I did not find the hematogenous pigment described by Krölling (1931) for G. rufifrons. There is no trophoblastic invasion of maternal vessels.
10)
Endometrium
No true decidua forms. Indeed, the endometrial stroma is fibrous-appearing,
with many distended glands. As is seen in earlier photographs shown above,
the endometrium interdigitates closely with the villous structures. In
between the caruncles, the endometrial glands are widely open and no decidual
transformation has taken place. Sections of the endocervical canal in
the immature uterus described above show the great abundance of cervical
mucus that is so characteristic of intact uteri.
11) Various features
There is no subplacenta and no other special regions are noteworthy.
12)
Endocrinology
Neumann et al. (2002) provided data that showed the stability of progesterone
in fecal samples from Dama gazelles and other taxa. It allows determination
of pregnancy and cycles. Thus, Pickard et al. (2001) studied the estrous
cycles of seven Mhorr gazelles from fecal samples. It was 18.62 days long.
The fetal ovaries of the specimen shown above were packed with primordial
germ cells and only a rare follicle with granulose cell investment had
formed in the ovarian centers.
We have undertaken extensive studies of the chromosome constitution of most available Antilopinae, including Mhorr gazelles (Effron et al., 1976; Benirschke, 1985). The most detailed study of Mhorr gazelles (55 specimens), however, comes from Nombela et al. (1990). It was conducted in the captive population of Almeira, Spain. Chromosome numbers of 2n=38 and 2n=39 were identified in that population. The chromosomal variation of G. dama and its relatives found by us is between 2n=38 and 2n=40, as was true of Dama gazelles studied by Vassart et al. (1995). That group of investigators published the best banding patterns of a variety of antilopini. Their contribution needs to be studied for broader conclusions. Vassart et al. (1993) described an unusual karyotype in a female Dama gazelle with four autosomal fusions, thus further documenting the complexity of Robertsonian translocations in Gazellinae.
One of the major features of numerous African gazelles is the Robertsonian fusion of an X-chromosome with an autosome. Thus, females may have two compound X-chromosomes, while males have one compound X-chromosome plus the corresponding autosome, and a Y-chromosome. Therefore, many males have one more chromosome, numerically-speaking. In all these gazelles, the autosome is the same, as identified by banding and other studies. This, we postulated, must be due to the origin (a long time ago) of these antelopes from a single ancestor that started with this fusion chromosome. Some other African antelopes do not share this feature (Effron et al., 1976). Subsequent to this event, various other Robertsonian fusions have taken place, mostly among autosomes. Thus, the different chromosome numbers of Dama gazelles may be variations that coincide with subspecies development, as Nombela et al. (1990) discussed extensively. These authors were also anticipating breeding the specimens with like chromosome numbers electively but have not yet published their results. In Mhorr gazelles an additional Y/autosome fusion is found, as is true of other African antelopes. The picture is indeed quite confusing and not completely resolved at this time. Nevertheless, as Vassart et al. (1995) reviewed, hybrid sterility can be expected when animals with monobrachial Robertsonian fusions mate. Unfortunately, the initial genetic analysis undertaken (cytochrome b) has not yet resolved the complex questions of speciation (Matthee & Robinson, 1999).
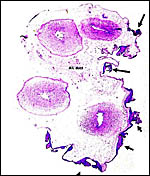
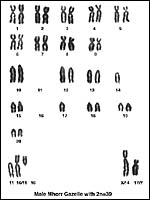 |
Karyotype of male Mhorr gazelle with 2n=39. |
 |
Karyotype of female Mhorr gazelle with 2n=39. |
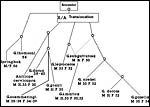 |
Diagram of postulated chromosomal evolution in some gazelles. |
I am not aware of any immunological studies conducted in this species.
15)
Pathological features
Griner (1983) listed trauma and malnutrition of young as the main causes
of mortality. Malignant catarrhal fever may also occur (Heuschele et al.,
1984). Occasional tumors have been observed in Dama gazelles. Thus, Schmidt & Fletcher (1979) reported on an adrenal adenoma of a 12 year old
animal with a 6x8 cm hemorrhagic mass that contained a 3x4 cm adenoma.
Martin et al. (1985) described a cementoblastoma removed from the maxilla
of a 6 y.o. gazelle that had a facial swelling for two years. The animal
recovered and subsequently bore an infant.
Drüwa (1985) provided much additional information on causes of death
(trauma, infections, "colds") and parasites. The immature specimen
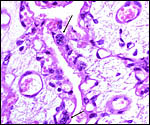
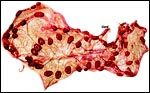
described above had a large peritubal cyst.
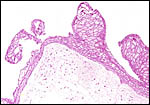
 |
The central ovoid structure is the ovary with corpus luteum, above is the large, thin-walled peritubal cyst. |
I am not aware of any physiological studies conducted in this species other than the elaboration of hematological norms provided by Pospisil et al. (1984), and semen studies. Holt et al. (1996) collected semen by electroejaculation and provided information on its storage. They used semen for artificial insemination and obtained several pregnancies in Mhorr gazelles but had only one survivor. Cassinello et al. (1998) also provided information on semen collected by electroejaculation. They described numerous spermatozoal abnormalities in Mhorr gazelle semen. Abaigar et al. (1999; 2001) used a sophisticated pattern analysis for the evaluation of sperm from Mhorr gazelles and boars. In their second publication these authors concentrated on the characteristics of Mhorr gazelles because of their small founder population (n=12). Gomendio et al. (2000) studied semen quality of three gazelle species (including the Dama gazelle) and concluded that inbreeding affects semen quality.
17)
Other resources
Numerous cell strains from animals at the San Diego Zoo are available
for study by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
The weight of only this one term placenta is recorded and the length of
a term umbilical cord is unknown.
Acknowledgement
The animal photographs in this chapter were taken at the San Diego Zoo.
I appreciate very much the help of the pathologists at the San Diego Zoo.
References
Abaigar, T., Holt, W.V., Harrison, R.A. and del Barrio, G.: Sperm subpopulations
in boar (Sus scrofa) and gazelle (Gazella dama mhorr) semen
as revealed by pattern analysis of computer-assisted motility assessments.
Biol. Reprod. 60:32-41, 1999.
Abaigar, T., Cano, M., Pickard, A.R. and Holt, W.V.: Use of computer-assisted sperm motility assessment and multivariate pattern analysis to characterize ejaculate quality in Mohor gazelles (Gazella dama mhorr): effects of body weight, electroejaculation technique and short-term semen storage. Reproduction 122:265-273, 2001.
Benirschke, K.: The genetic management of exotic animals. Sympos. Zool. Soc. London 54:71-87, 1985.
Cassinello, J., Abaigar, T., Gomendio, M. and Roldan, E.R.: Characteristics of the semen of three endangered species of gazelles (Gazella dama mhorr, G. dorcas neglecta and G. cuvieri). Reprod. Fertil. 113:35-45, 1998.
Dolan, J.M.: The Mhorr gazelle (Gazella dama mhorr) (Bennett, 1833): A case for captive propagation. J. Zoo Anim. Med. 12:114-117, 1981.
Drüwa, P.: Die Damagazelle (Gazella dama ssp. Pallas, 1767), einige Beiträge zur allgemeinen Biologie, Haltung und Zucht im Zoologischen Garten. Zool. Garten 55:1-28, 1985.
Effron, M., Bogart, M.H., Kumamoto, A.T. and Benirschke, K.: Chromosome studies in the mammalian subfamily Antilopinae. Genetica 46:419-444, 1976.
Eigener, W. and Schliemann, H.: Anmerkungen zu der irrtümlicherweise als Mhorrgazelle bezeichneten Gazella dama aus der spanischen Sahara. Zool. Garten 53:313-316, 1983.
Gomendio, M., Cassinello, J. and Roldan, E.R.: A comparative study of ejaculate traits in three endangered ungulates with different levels of inbreeding: fluctuating asymmetry as an indicator of reproductive and genetic stress. Proc. Roy. Soc. London B Biol. Sci. 267:875-882, 2000.
Grettenberger, J.F. and Newby, J.E.: The status and ecology of the Dama gazelle in the A?r and Ténéré National Nature Reserve, Niger. Biol. Conservation 38:207-216, 1986.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Heuschele, W.P., Oosterhuis, J., Anderson, M.P., Swansen, M. and Fletcher, H.R.: Malignant catarrhal fever in wild ruminants. Chapter 25 (pp. 296-308). In, One Medicine, Springer-Verlag, NY, 1984.
Holt, W.V., Abaigar, T. and Jabbour, H.N.: Oestrous synchronization, semen preservation and artificial insemination in the Mohor gazelle (Gazella dama mhorr) for the establishment of a genome resource bank programme. Reprod. Fertil. Devel. 8:1215-1222, 1996.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbook 32:159-169, 1993.
Krölling, O.: Über den Bau der Antilopenplazentome. Z. Mikrosk. Anat. Forsch. 27:216-232, 1931.
Lange,
J.: Ein Beitrag zur systematischen Stellung der Spiegelgazellen (Genus
Gazella Blainville, 1816. Subgenus Nanger Lataste, 1885).
Z. Säugetierk. 36:1-18, 1971.
Martin, H.D., Turner, T., Kollias, G.V., Lin, S.L., Heard, D.J. and Jacobson,
E.: Cementoblastoma in a Dama gazelle. J. Amer. Vet. Med. Assoc. 187:1246-1247,
1985.
Matthee, C.A. and Robinson, T.J.: Cytochrome b phylogeny of the family bovidae: Resolution within the alcelaphine, antilopini, Neotragini, and tragelaphini. Mol. Phylogenet. Evol. 12:31-46, 1999.
Neumann, G., Gottschalk, J., Eulenberger, G. and Grun, E.: The stability in progesterone in feces of different wild animal species kept in a zoological garden (in German). Dtsch. Tierärztl. Wochenschr. 109:245-249, 2002.
Nombela, J.J.A., Murcia, C.R., Abaigar, T. and Vericad, J.R.: GTG-banded karyotype of Gazella dama mhorr Bennett, 1833. Cytogenetic relationship with other members of the subgenus Nanger. Z. Säugetierk. 55:194-201, 1990.
Perez, M.C.: Revision der Systematik von Gazella (Nanger) dama. Zeitschr. Kölner Zoo 27:103-107, 1984.
Pickard, A.R., Abaigar, T., Green, D.I., Holt, W.V. and Cano, M.: Hormonal characterization of the reproductive cycle and pregnancy in the female Mohor gazelle (Gazella dama mhorr). Reproduction 122:571-580, 2002.
Pospisil, J., Kase, F., Vahala, J. and Mouchova, I.: Basic haematological values in antelopes - III. The Reduncinae and the Antelopinae. Comp. Biochem. Physiol. A 78:809-813, 1984.
Schmidt, R.E. and Fletcher, K.C.: Adrenal cortical adenoma in a dama gazelle (Gazella dama). J. Wildl. Dis. 15:299-301, 1979.
Vassart, M., Greth, A., Durand, V. and Cribiu, E.P.: An unusual Gazella dama karyotype. Ann. Genet. 36:117-120, 1993.
Vassart, M., Séguéla and Hayes, H.: Chromosomal evolution in gazelles. J. Hered. 86:216-227, 1995.
Wiesner, H. and Muller, P.: On the reintroduction of the Mhorr gazelle in Tunisia and Morocco. Naturwissensch. 85:553-555, 1998.