| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: May 16, 2004.. |
Mandrillus sphinx
Order: Primates
Family: Cercopithecidae
1) General Zoological Data
The name mandrill, suggests Gotch (1979), derives from “man” and “drill”, Western African names for baboons. The most closely related species, the drill ( Mandrillus leucophaeus) is larger and perhaps even less common. Tutin et al. (1997) observed a variety of diurnal primates in the wild at Lope , Gabon , especially for their food preferences. Fruits were the primary resource, except during their scarcity periods in the dry season. Mandrills inhabit primarily Western Africa/Gabon and Cameroon (Matthews & Matthews, 2002).
 |
Mandrill at San Diego Zoo. |
2) General Gestational Data
Wickings & Dixson (1992) observed the breeding patterns of a group of free-ranging mandrills in Gabon and determined that sexual maturity of females begins between 2.75 and 4.5 years and first delivery occurs at age 3.25 years. The gestation period varied between 152 and 176 days with an interbirth interval of 11-15 months. They also reported that males (30-35 kg at maturity) developed more slowly than females (10-15 kg). Generally singletons are produced after a 167 +/- day gestation. Singletons are the rule but, while occasional baboon twins have been reported, no mandrill twins are listed in the accumulated data collected by Geissmann (1990).This full term newborn whose placenta was available weighed 825 g. The placenta weighed 150 g and had a 27 cm long umbilical cord with three vessels and no ducts.
3) Implantation
The early stages of embryogenesis and placentation are presumably essentially the same as in baboons (Hendrickx, 1971) and these are reviewed in the chapter on macacs (see “rhesus monkey” chapter).
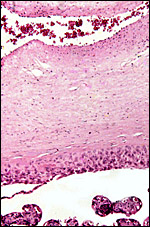
4) General Characterization of the Placenta
As in baboons, the mandrill placenta consists of a single disk. The placenta resembles closely that of baboons and rhesus monkeys, as far as their villous structure is concerned. Cotyledons are not grossly visible. Mandrills have a villous, hemochorial type of placentation.
This placenta comes from a term gestation with neonatal demise and was the product of a female that died from disseminated tuberculosis and weighed 150 g. Placenta and fetus had no evidence of tuberculosis.
5) Details of fetal/maternal barrier
The fetal capillaries abut and occasional indent the trophoblastic surface. Despite this being a term gestation, cytotrophoblast (“Langhans' cells”) was occasionally still visible beneath the syncytial cover of the villi. There were no mitoses. The villi are composed of connective tissue and contain scattered macrophages (Hofbauer cells).
6) Umbilical cord
The umbilical cord of this placenta was inserted eccentrically and was 27 cm long. It contained two arteries and one vein. There were no ducts and no twists of the cord. The surface was composed of a thin layer of amnionic epithelium. The Hyrtl anastomosis is much the same as in other cercopithecid and human placentas (see chapter on rhesus monkey; Houston & Hendrickx, 1968).
7) Uteroplacental circulation
See chapter on rhesus monkey in which much the same vascularization prevails.
8) Extraplacental membranes
As is true of other cercopithecids, the free membranes (the chorion laeve) contain no atrophying villi, as is the case in human gestations. There is merely decidua capsularis which is separated from the chorion by a few trophoblastic cells. The chorion leave contained no blood vessels. The amnion has a single layer of epithelial cells and a glassy, homogeneous layer upon which they rest. Macrophages are found in the spongy layer of the amnion.9) Trophoblast external to barrier
Cytotrophoblast (“extravillous trophoblast”) infiltrates the deciduas basalis and its vessels. I have no preparations of the uterus to determine whether there is Myometrial invasion.
10) Endometrium
There is typical decidualization of the mandrill endometrium during pregnancy. This has recently been studied in baboons with the recognition of the “pivotal role” in extracellular matrix remodeling by matrix metalloproteinases (Strakova et al., 2003). Moreover, there is extensive infiltration of round cells in the developing decidua that has been studied in the (probably similar reaction of) rhesus monkey (Sluvkin et al., 2004). It is presumed that these cells (NK cells and T-cells) are of importance in immune regulation of the infiltrating trophoblast.
Fazleabas et al. (1999) created the endometrial plaque of implantation in baboons by hCG administration and determined the endometrial changes then induced by additional steroid application. Pepe & Albrecht (1995) provided an extensive review of adrenal steroids (fetal and maternal) in catarrhine pregnancies and their modulation by placental enzymes.
11) Various features
No special features are recognized.
12) Endocrinology
Wickings & Dixson (1992) determined testosterone values of adult males to be 8.17 ng/ml. Siler-Khodr et al. (2003) examined the effect of GnRH on progesterone release from granulose cell cultures of baboons. The possible regulation of chorionic somatomammotropin release from syncytiotrophoblast by estrogens was studied in baboons by Musicki et al. (2003). They found suppression in the first trimester.
13) Genetics
Mandrills, like baboons, have 42 chromosomes. Chromosome 13 has a major secondary constriction proximal to the centromere. Rubio-Goday et al. (1976) compared the G-banding patterns of Mandrillus (P.) sphinx chromosomes with that of Macaca fascicularis whose chromosome number is also 2n=42. Only one pair showed a small pericentric inversion polymorphism. Lucotte & Jouventin (1980) compared electrophoretic patters between Mandrill and Drill ( Mandrillus leucophaeus ) and detected 30% differences of their bands. An extensive study of DNA fingerprints (GTG 5 short tandem repeat sequences and minisatellites) was carried out at the Franceville Center in Gabon by Wickings (1995). This colony has had a spectacular breeding success and the breeding hierarchy was ascertained by this methodology. Gray (1972) lists a number of hybrids. Mandrillus sphinx and Mandrillus leucophaeus have hybridized many times, and several Papio hybrids have also been reported. Also, a few hybrids with macacs and mangabeys are on record but most did not survive and nothing about the possible fertility of hybrids is known.
Divergence found in the composition of the cytochrome b gene of known-origin mandrills allowed identification of populations that were separated by a large river in Gabon (Telfer et al., 2003). This also correlated with the distribution of two immunodeficiency viruses of these populations.
 |
Karyotype of male mandrill. |
14) Immunology
The immune response to infection with irradiated Loa loa larvae was reported by Ungeheuer et al. (2000).
15) Pathological features
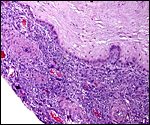
Placental infarcts are common in the placentas of cercopithecids and were also found in this placenta. They are not accompanied by symptoms suggesting pre-eclampsia, as would be the case in human gestations. While thrombosed decidual vessels can be seen in such placental floors, their walls are not altered as is the case in humans with similar pathology.
Remarkably, Johnson et al. (1998) reported a fatal case of coccidioidomycosis in a mandrill colony held in Texas . Of greater interest perhaps is the designation of a free-living ameba as Balamuthia mandrillaris (Visvesvara et al., 1993; 1990) which was first identified in a mandrill at San Diego Zoo and has features that differ from those of unlike Acanthamoeba and Naegleria known to cause encephalitis. It has also caused encephalitis in gorilla and Colobus monkeys (Rideout et al., 1997) and humans (Denney et al., 1997). Its genotypic characteristics were studied by Booton et al. (2003). Paratuberculosis due to Mycobacterium avium infection was the cause of death in a 2 ½ y.o. mandrill reported by Zwick et al. (2002). Echinococcosis caused death of a mandrill reported by Boever & Britt (1975). Encephalomyocarditis virus infection was fatal to three mandrills and several other animals at the Taronga zoo in Sydney , Australia (Reddacliff et al., 1997). Mandrills are also carriers of lentiviruses (SIV) (Pandrea et al., 2003) and the virus is transmitted to humans in the African bushmeat pandemic (Wolfe et al., 2004).
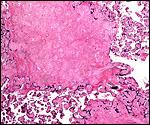 |
Typical old placental infarct in the placenta of a mandrill. |
16) Physiologic data
Lin et al. (1988) determined the structure of mandrill hemoglobin chains and compared them with other cercopithecids. They thus suggested placement of mandrills between macacs and baboons. Peinado et al. (1996) published hematologic values of mangabeys and mandrills and found them to be essentially similar to other cercopithecids. Zachos et al. (2004) deprived (or supplemented) gestations of estrogens and showed a decrease of fetal oocytes in the deprived animals. They proposed that the microvillous surface of oocyte development is regulated by estrogens.
17) Other resources
Cell strains of fibroblast cultures are available from CRES at San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu .
18) Other remarks – What additional Information is needed?
Definitive stages of mandrill implantation and their early development are missing. Also, little specific endocrine data are published.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society of San Diego.
References
Boever, W.J. and Britt, J.: Hydatid disease in mandrill baboons. J. Amer. Vet. Med. Assoc. 167:619-621, 1975.
Booton, G.C., Carmichael , J.R., Visvesvara, G.S., Byers, T.J. and Fuerst, P.A.: Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rDNA genes. Amer. J. Trop. Med. Hyg. 68:65-69, 2003.
Denney, C.F., Iragui, V.J., Uber-Zak, L.D., Karpinski , N.C. , Ziegler, E.J., Visvesvara, G.S. and Reed, S.L.: Amebic meningoencephalitis caused by Balamuthia mandrillaris : case report and review. Clin. Infect. Dis. 25:1345-1348, 1997.
Fazleabas, A.T., Donnelly, K.M., Srinivasan, S., Fortman, J.D. and Miller, J.B.: Modulation of the baboon ( Papio anubis ) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc. Natl. Acad. Sci. USA 96:2543-2548, 1999.
Geissmann, T.: Twinning frequency in catarrhine primates. Human Evol. 5:387-396, 1990.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole , Dorset , 1979.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2 nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough , England , 1972.
Hendrickx, A.G.: Embryology of the Baboon. University of Chicago Press, Chicago, 1971.
Houston , M.L. and Hendrickx, A.G.: Observations on the vasculature of the baboon placenta ( Papio sp.) with special reference to the transverse communicating artery. Folia Primatol. 9:68-77, 1968.
Johnson, J.H., Wolf, A.M., Edwards, J.F., Walker , M.A., Homco, L., Jensen, J.M., Simpson, B.R. and Taliaferro, L.: Disseminated coccidioidomycosis in a mandrill baboon ( Mandrillus sphinx ): a case report. J. Zoo Wildl. Med. 29:208-213, 1998.
Lin, H.X., Kleinschmidt, T., Braunitzer, G. and Goltenboth, R.: The primary structure of the mandrill ( Mandrillus sphinx , Primates) hemoglobin. Biol. Chem. Hoppe Seyler 369:209-216, 1988.
Lucotte, G. and Jouventin, P.: Electrophoretic distance between the Mandrill and the Drill. (In French). Ann. Genet. 23:46-48, 1980.
Matthews, A. and Matthews, A.: Distribution, population density, and status of sympatric cercopithecids in the Campo-Ma'an area, Southwestern Cameroon . Primates 43:155-168, 2002.
Musicki, B., Pepe, G.J. and Albrecht, E.D.: Functional differentiation of the placental syncytiotrophoblast: Effect of estrogen on chorionic somatomammotropin expression during early primate pregnancy. J. Clin. Endocrinol. Metab. 88:4316-4323, 2003.
Pandrea, I. , Onanga, R., Kornfeld, C., Rouquet, P., Bourry, O., Clifford, S., Telfer, P.T., Abernathy, K., White, L.T., Ngari, P., Muller-Trutwin, M., Roques, P., Marx, P.A., Simon, F. and Apetrei, C.: High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx . Virology 317:119-127, 2003.
Peinado, V.I., Celdran, J.F., Viscor, G. and Palomeque, J.: Hematology and serum chemistry in the white-crowned mangabey ( Cercocebus torquatus lunulatus ) and in the mandrill ( Mandrillus sphinx ). J. Med. Primatol. 25:282-286, 1996.
Pepe, G.J. and Albrecht, E.D.: Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Reviews 16:608-648, 1995.
Reddacliff, L.A. , Kirkland , P.D., Hartley, W.J. and Reece, R.L.: Encephalomyocarditis virus infection in an Australian zoo. J. Zoo Wildl. Med. 28:153-157, 1997.
Rideout, B.A., Gardiner, C.H., Stalis, I.H., Zuba, J.R., Hadfield, T. and Visvesvara, G.S.: Fatal infections with Balamuthia mandrillaris (a free-lining amoeba) in gorillas and other Old World primates. Vet. Pathol. 34:15-22, 1997.
Rubio-Goday, A., Caballin, M.R., Garcia-Caldes, M. and Egozcue, J.: Comparative study of the banding patterns of the chromosomes of cercopithecidae. I. Subfamily Papinae: Macaca fascicularis and Papio sphinx . Folia Primatol. 26:306-309, 1976.
Siler-Khodr, T.M., Grayson, M. and Eddy , C.A. : Action of gonadotropin-releasing hormone II on the baboon ovary. Biol. Reprod. 68:1150-1156, 2003.
Sluvkin, I.I., Breburda, E.E. and Golos, T.G.: Dynamic changes in primate endometrial leukocyte populations: Differential distribution of macrophages and natural killer cells at the Rhesus monkey implantation site and in early pregnancy. Placenta 25:297-307, 2004.
Strakova, Z., Szmidt, M., Srisuparp, S. and Fazleabas, A.T.: Inhibition of matrix metalloproteinases prevents the synthesis of insulin-like growth factor binding protein-1 during decidualization in the baboon. Endocrinology 144:5339-5446, 2003.
Telfer, P.T., Souquiere, S., Clifford, S.L., Abernethy, K.A., Bruford, M.W., Disotell, T.R., Sterner, K.N., Roques, P., Marx, P.A. and Wickings, E.J.: Molecular evidence for deep phylogenetic divergence in Mandrillus sphinx . Mol. Ecol. 12:2019-2024, 2003.
Tutin, C.E., Ham, R.M., White, L.J. and Harrison , M.J.: The primate community of the Lope Reserve, Gabon : diets, responses to fruit scarcity, and effects on biomass. Amer. J. Primatol. 42:1-24, 1997.
Ungeheuer, M., Elissa, N., Morelli, A., Georges, A.J., Deloron, P., Debre, P., Bain, O. and Millet, P.: Cellular responses to Loa loa experimental infection in mandrills ( Mandrillus sphinx ) vaccinated with irradiated infective larvae. Parasite Immunol. 22:173-183, 2000.
Visvesvara, G.S., Martinez , A.J., Schuster, F.L., Leitch, G.L., Wallace, S.V., Sawyer, T.K. and Anderson , M.: Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J. Clin. Microbiol. 28:2750-2756, 1990.
Visvesvara, G.S., Schuster, F.L. and Martinez , A.J.: Balamuthia mandrillaris , N.G., N.Sp., agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 40:504-514, 1993.
Wickings, E.J.: Genetic self-management in a captive colony of mandrills ( Mandrillus sphinx ) as revealed by DNA minisatellite fingerprints. Electrophoresis 16:1678-1683, 1995.
Wickings, E.J. and Dixson, A.F.: Development from birth to sexual maturity in a semi-free-ranging colony of mandrills ( Mandrillus sphinx ) in Gabon . J. Reprod. Fertil. 95:129-138, 1992.
Wolfe , N.D. , Switzer, W.M., Carr, J.K., Bhullar, V.B., Shanmugam, V., Tamoufe, U., Prosser, A.T., Torimiro, J.N., Wright, A., Mpoudi-Ngole, E., McCutchan, F.E., Burx, D.L., Folks, T.M., Burke, D.S. and Heneine, W.: Naturally acquired simian retrovirus infections in central African hunters. Lancet 363:932-937, 2004.
Zachos , N.C. , Billiar, R.B., Albrecht, E.D. and Pepe, G.J.: Regulation of oocyte microvilli development in the baboon fetal ovary by estrogen. Endocrinology 145:959-966, 2004.
Zwick, L.S., Walsh, T.F., Barbiers, R., Collins, M.T., Kinsel, M.J. and Murnane, R.D.: Paratuberculosis in a mandrill ( Mandrillus sphinx ). J. Vet. Diagn. Invest. 14:326-328, 2002.