| |
Spontaneous abortions occur but are perhaps not so common as seen in human
gestations. This is particularly so in breeding colonies of Primate Research
Centers. Wilson (1972) reviewed this topic extensively. Around 10-15% of
gestations that occurred in Primate Research Centers abort spontaneously
or end in premature delivery. Many more do so when the females were freshly
imported. Similarly, Hertig et al. (1971) reported that nearly one half
of newly imported pregnant animals had either abortions or premature deliveries.
Most were due to common infections and/or measles infection. In contrast
to human studies of abortions, a majority of which are due to trisomies
or other chromosomal errors, such investigations have apparently not been
done in rhesus monkey colonies.
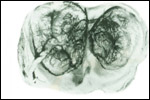
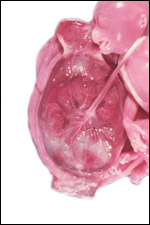
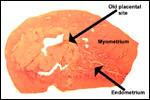
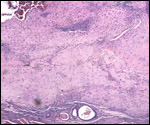
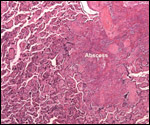
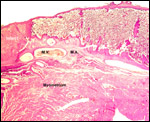
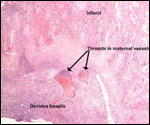
Placenta previa, abruptio placentae, infarcts and stillbirths with fetus
papyraceus all have been recorded in rhesus monkeys (Myers, 1972). Indeed,
Myers asserted that most of the anomalies or pathologic conditions seen
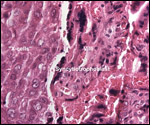
in human placentas may be observed in rhesus monkeys. Endometriosis, adenomyosis
and endometrial anomalies are some other pathologic features studied in
rhesus monkeys.
Structural abnormalities of neonatal primates have been studied by Wilson
(1972). A variety of congenital anomalies have been identified, but apparently
fewer than found in humans. Importantly, the same author clearly identified
the cause of phocomelia following a single dose of thalidomide. Several
of those animals are depicted in his report.
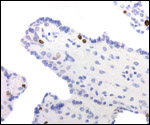
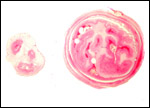
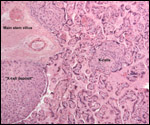
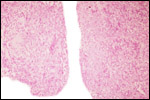
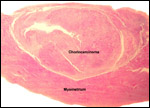
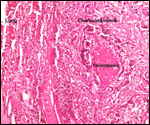
Several extrauterine choriocarcinomas have been reported, even in juvenile catarrhine monkeys. They may have arisen from germ cells or stem cells; alternatively they are associated with a teratoma. Their apparent relative frequency is nevertheless surprising and they can be quite destructive. The last-cited authors have used very modern tools for their exploration. (Farman et al. 2005; Giusti, et al. 2005; Marbaix et al., 2008; Moore et al., 2003; Toyosawa et al. 2000; Yamamoto et al. 2007).
16)
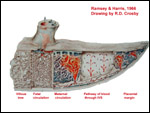
Physiologic data
Physiologic data on blood pressure and pulse rate of fetus and mother
in pregnant rhesus monkeys under anesthesia were reported by Misenhimer
& Ramsey (1970. In addition to that report, a wealth of information
has been accumulated in fetal and placental physiology of rhesus gestations.
Access to this literature can be achieved through the comprehensive book
of Ramsey and Donner (1980). Some information on the vasculature is summarized
above.
17)
Other resources
The Regional Primate Research Centers of the USA, several foreign research
centers, and industrial companies have large holdings of rhesus monkeys.
Most are now bred in captivity, very few are being imported. And the zoological
parks of the world, of course, house large numbers of these animals and
more commonly some of the related species. There is much expertise in
breeding and pathology in these agencies.
18)
Other remarks - What additional Information is needed?
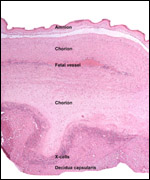
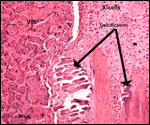
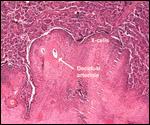
One of the more interesting future studies should be on the regulation
of the "X-cells", the extravillous trophoblast. Not only does
it achieve the implantation but also, it modifies the blood vessels of
the endometrium and produces a specific protein whose function is unknown
so far. The means by which MHC is modulated so as to prevent rejection,
the exploration of syncytin and syncytium formation will be of interest.
Observations on intrauterine mobility and its effect on spiraling (not
described in rhesus) of the cord should be made. Do MZ twins occur and
how is their placenta structured.
Acknowledgement
Most of the animal photographs in these chapters come from the Zoological
Society of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Arts, N.F.Th. and Lohman, A.H.M.: An injection=corrosion study of the
fetal and maternal vascular systems in the placenta of the rhesus monkey.
Europ. J. Obstet. Gynecol. Reprod. Biol. 4:133-141, 1974.
Atkinson,
L.E., Hotchkiss, J., Fritz, G.R., Surve, A.H., Neill, J.D. and Knobil,
E.: Circulating levels of steroids and chorionic gonadotropin during pregnancy
in the rhesus monkey, with special attention to the rescue of the corpus
luteum in early pregnancy. Biol. Reprod. 12:335-345, 1975.
Bartelmez,
G.W.: Cyclic changes in the endometrium of the rhesus monkey (Macaca
mulatta). Contrib. Embryol. Carnegie Institution # 227. 34:101-144,
1951.
Beck, A.J. and Beck, F.: The origin of intra-arterial cells in the pregnant
uterus of the macaque (Macaca mulatta). Anat. Rec. 158:111-114,
1967.
Benirschke,
K. and Kaufmann, P.: The Pathology of the Human Placenta. 4th ed. Springer-Verlag,
NY, 2000.
Boyson,
J.E., Iwanaga, K.K., Golos, T.G. and Watkins, D.I.: Identification of
a novel MHC class I gene, Mamu-AG, expressed in the placenta of a primate
with an inactivated G locus. J. Immunol. 159:3311-3321, 1997.
Bronson,
R., Volk, T.L. and Ruebner, B.H.: Involution of placental site and corpus
luteum in the monkey. Amer. J. Obstet. Gynecol. 113:70-75, 1972.
Bunton,
T.E.: Incidental lesions in nonhuman primate placentae. Vet. Pathol. 23:431-438,
1986.
Chez,
R.A., Schlesselman, J.J., Salazar, H. and Fox, R.: Single placentas in
the Rhesus Monkey. J. med. Primatol. 1:230-240, 1972.
Enders,
A.C. and King, B.F.: Formation and differentiation of extraembryonic mesoderm
in the rhesus monkey. Amer. J. Anat. 181:327-340, 1988.
Enders,
A.C. and Schlafke, S.: Differentiation of the blastocyst of the rhesus
monkey. Amer. J. Anat. 162:1-21, 1981.
Enders,
A.C., Hendrickx, A.G. and Binkerd, P.E.: Abnormal development of blastocysts
and blastomeres in the rhesus monkey. Biol. Reprod. 26:353-366, 1982.
Fa,
J.E.: The genus Macaca: a review of taxonomy and evolution. Mamm.
Rev. 19:45-81, 1989.
Farman, , C.A.: Benirschke, K., Horner, M. and Lappin, P: Ovarian choriocarcinoma in a rhesus monkey associated with elevated serum chorionic gonadotropin levels. Vet. Pathol. 42:226-229, 2005.
Fitch, H., Coen, R., Lindburg, D., Robinson, P., Hajek, P. Hesselink, J. and Crutchfield, S.: Cerebral palsy in a macaque monkey. Amer. J. Primatol. 14:181-187, 1988.
Galton,
M.: DNA content of placental nuclei. J. Cell. Biol. 13:183-191, 1962.
Geissmann,
T.: Twinning frequency in catarrhine primates. Human Evolution 5:387-396,
1990.
Giusti, A.M.F., Terron, A., Belluco, S., Scanziani, E. and Carccangiu, M.L.: Ovarian epithelioid trophoblastic tumor in a cynomolgus monkey. Vet. Pathol. 42:223-226, 2005.
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Gruenwald,
P.: Lobular structure of hemochorial primate placentas, and its relation
to maternal vessels. Amer. J. Anat. 136:133-152, 1973.
Hendrickx,
A.G.: Embryology of the Baboon. University of Chicago Press, Chicago,
1971.
Hertig,
A.T., King, N.W. and MacKey, J.: Spontaneous abortion in wild-caught rhesus
monkeys, Macaca mulatta. Labor. Animal Sci. 21:510-519, 1971.
Heuser,
C.H. and Streeter, G.L.: Development of the macaque embryo. Contrib. Embryol.
Carnegie Institution # 181. 29:9-55, 1941.
Hobson,
B.M.: Production of gonadotropin, oestrogens and progesterone by the primate
placenta. Adv. Reprod. Physiol. 5:67-102, 1971.
Hodgen,
G.D., Dufau, M.L., Catt, K.J. and Tullner, W.W.: Estrogens, progesterone
and chorionic gonadotropin in pregnant rhesus monkeys. Endocrinology 91:896-900,
1972.
Houston,
M.L. and Hendrickx, A.G.: Observations on the vasculature of the baboon
placenta (Papio sp.) with special reference to the transverse communicating
artery. Folia primatol. 9:68-77, 1968.
King,
B.F.: Developmental changes in the fine structure of rhesus monkey amnion.
Amer. J. Anat. 157:285-307, 1980.
King,
B.J.: Developmental changes in the fine structure of the chorion leave
(smooth chorion) of the rhesus monkey placenta. Anat. Rec. 200:163-175,
1981.
Knapp,
L.A., Lehmann, E., Piekarczyk, M.S., Urvater, J.A. and Watkins, D.I.:
A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase
chain reaction with sequence-specific primers and direct sequencing. Tissue
Antigens 50:657-661, 1997.
Knapp,
L.A., Cadavid, L.F. and Watkins, D.I.: The MHC-E locus in the most ancient
and well-conserved of all known primate class I histocompatibility genes.
J. Immunol. 160:180-196, 1998.
Kuroda,
M.C., Schmitz, J.E., Barouch, D.H., Craiu, A., Allen, T.M., Sette, A.,
Watkins, D.I., Forman, M.A. and Letvin, N.L.: Analysis of gag-specific
cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys by cell staining
with tetrameric MHC Class I/peptide complex. J. Exp. Med. 187:1373-1381,
1998.
Landsteiner,
K. and Wiener, A.S.: An agglutinable factor in human blood recognized
by immune sera for rhesus blood. Proc. Soc. Exp. Biol. Med. 43:223-224,
1940.
Lewis,
J. and Hertz, R.: Effects of early embryectomy and hormonal therapy on
the fate of the placenta in pregnant rhesus monkeys. Proc. Soc. Exp. Biol.
Med. 123:805-809. 1966.
Lindburg,
D.G.: The rhesus monkey in north India: an ecological and behavioral study.
Primate Behav. 2:1-106, 1971.
Lindburg,
D.G., ed.: The macaques: studies in ecology, behavior, and evolution.
Van Nostrand Reinhold, NY, 1980.
Lindsey,
J.R., Wharton, L.R., Woodruff, J.D. and Baker, H.J.: Intrauterine choriocarcinoma
in a rhesus monkey. Pathol. Vet, 6:378-384, 1969.
Luckett,
W.P.: The fine structure of the placental villi of the rhesus monkey (Macaca
mulatta). Anat. Rec. 167:141-164, 1970.
Maddox,
D.E., Kephart, G.M., Coulam, C.B., Butterfield, J.H., Benirschke, K. and
Gleich, G.J.: Localization of a molecule immunochemically similar to eosinophil
major basic protein in human placenta. J. Exp. Med.. 160:29-41, 1984.
Marbaix, E., Defrere, S., Ho Minh Duc, K., Lousse J.-C. and Dehoux, J.-P.: Non-gestational malignant placental site trophoblastic tumor of the ovary in a 4-year-old rhesus monkey. Vet. Pathol. 45:375-378, 2008.
Martin,
C.B., Ramsey, E.M. and Donner, M.W.: The fetal placental circulation in
rhesus monkeys demonstrated by radioangiography. Amer. J. Obstet. Gynecol.
95:943-947, 1966.
Migaki,
G., Benirschke, K., McKee, A.E. and Casey, H.W.: Trichomonal granuloma
of the pelvic cavity in a rhesus monkey. Vet. Path. 15:679?681, 1978.
Misenhimer,
H.R. and Ramsey, E.M.: The effect of anesthesia and surgery in pregnant
rhesus monkeys. Gynec. Invest. 1:105-114, 1970.
Moore, C.M., Hubbard, G.B., Leland, M.M., Dunn, B.G. and Best, R.G.: Spontaneous ovarian tumors in twelve baboons: a review of ovarian neoplasms in nonhuman primates. J. Med. Primatol. 32:48-56, 2003.
Myers,
R.E.: The gross pathology of the Rhesus Monkey placenta. J. Reprod. Med.
9:171-198, 1972.
Myers,
R.E.: The pathology of the rhesus monkey placenta. Pp. 221-260. In, Symposium
on the Use of Non-human Primates for Research on Problems of Human Reproduction.
Sukhumi, 1971. WHO Research & Training Centre on Human Reproduction,
Karolinska, Sweden, 1972.
Myers,
R.E.: Placental pathology. Chapter 85 in, Spontaneous Animal Models of
Human Diseases, Vol. 1, E.J. Andrews, B.C. Ward, and N.H. Altman eds.,
Academic Press, NY, 1979. pp. 208-209.
Naaktgeboren,
C. and Wagtendonk, A.M.v.: Wahre Knoten in der Nabelschnur nebst Bemerkungen
über Plazentophagie bei Menschenaffen. Z. Säugetierk. 31:376-382,
1966.
Nathanielsz,
P.W., Figueroa, J.P. and Honnebier, M.B.O.M.: In the rhesus monkey placental
retention after fetectomy at 121 to 130 days' gestation outlasts the normal
duration of pregnancy. Amer. J. Obstet. Gynecol. 166:1529-1535, 1992.
Novy,
M.J., Aubert, M.L., Kaplan, S.L. and Grumbach, M.M.: Regulation of placental
growth and chorionic somatomammotropin in the rhesus monkey: Effects of
protein deprivation, fetal anencephaly, and placental vessel ligation.
Amer. J. Obstet. Gynecol. 140:552-562, 1981.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Panigel,
M.: Comparative anatomical, physiological and pharmacological aspects
of placental permeability and haemodynamics in the non-human primate placenta
and in the isolated perfused human placenta. In, The Foeto-Placental Unit.
Pp.279-295, Excerpta Medica Internat. Congress Series # 183, Milan 1968.
Pepe,
G.J. and Albrecht, E.D.: Actions of placental and fetal adrenal steroid
hormones in primate pregnancy. Endocrine Reviews 16:608-648, 1995.
Pierce,
G.B., Midgley, A.R. and Beals, T.F.: An ultrastructural study of differentiation
and maturation of trophoblast of the monkey. Laboratory Invest. 13:451-464,
1964.
Raio,
L., Ghezzi, F., DiNaro, M. Franchi, M., Balestreri, D., Durig, P. and
Schneider, H.: In-utero characterization of the blood flow in the Hyrtl
anastomosis. Placenta 22:597-601, 2001.
Ramsey,
E.M. and Harris, J.W.S.: Comparison of Uteroplacental vasculature and
circulation in the rhesus monkey and man. Contrib. To Embryol. # 261.
Carnegie Inst. Washington Publ. # 625. Vol. 38:59-70, 1966.
Ramsey,
E.M., Martin, C.B., McGaughey, H.S., Kaiser, I.H. and Donner, M.W.: Venous
drainage of the placenta in rhesus monkeys: Radiographic studies. Amer.
J. Obstet. Gynecol. 95:948-955, 1966.
Ramsey,
E.M.: Evaluation of Macaca mulatta as an experimental model for
studies of primate reproduction. In, Medical Primatology, part 1, pp.
308-316, 1972. Karger, Basel.
Ramsey,
E.M., Houston, M.L. and Harris, J.W.S.: Interactions of the trophoblast
and maternal tissues in three closely related primate species. Amer. J.
Obstet. Gynecol. 124-647-652, 1976.
Ramsey,
E.M., Chez, R.A. and Doppman, J.L.: Radioangiographic measurement of the
internal diameters of the Uteroplacental arteries in rhesus monkeys. Amer.
J. Obstet. Gynecol. 135:247-251, 1979.
Ramsey,
E.M. and Donner, M.W.: Placental Vasculature and Circulation. Anatomy,
Physiology, Radiology, Clinical Aspects. Atlas and Textbook. W.B. Saunders
Company, Philadelphia, 1980.
Richart,
R.: Studies of placental morphogenesis. I. Radioautographic studies of
human placenta utilizing tritiated thymidine. Proc. Soc. Exp. Biol. Med.
106:829-831, 1961.
Ruppenthal, G.C., Moore, C.M., Best, R.G., Walker-Gelatt, C.G., Delio, P.J. and Sackett, G.P.: Trisomy 16 in a pigtailed macaque (M. nemestrina) with multiple anomalies and developmental delays. Amer. J. Ment. Retard. 109:9-20, 2004.
Scott,
G.B.D.: Comparative Primate Pathology. Oxford University Press, 1992.
Socha,
W.W.: Blood groups of nonhuman primates. In, Comparative Biology. Vol.
1: Systematics, Evolution, and Anatomy. Pp. 299-333, A. R. Liss, 1986.
Spatz,
W.B.: Nabelschnur-Längen bei Insektivoren und Primaten. Z. Säugetierk.
33:226-239, 1968.
Starck,
D.: Vergleichende Anatomie und Evolution der Placenta. Verh. Anat. Gesellsch.
56th Versammlung, Zurich, 1959. Gustav Fischer Verlag, Jena, 1959. Pp.5-26.
Stock,
A.D. and Hsu, T.C.: Evolutionary conservatism in arrangement of genetic
material. A comparative analysis of chromosome banding between the rhesus
macaque (2=42, 84 arms) and the African green monkey (2n=60, 120 arms).
Chromosoma 43:211, 1973.
Torpin,
R.: Placentation in the rhesus monkey (Macaca mulatta). Obstet.
Gynecol. 34:410-413, 1969.
Toyosawa, K., Okimoto, K., Koujitani, T. and Kikawa, E.: Choriocarcinoma and teratoma in the ovary of a cynomolgus monkey. Vet. Pathol. 37:186-188, 2000.
Wagenen,
G.v., Catchpole, H.R., Negri, J. and Butzko, D.: Growth of the fetus and
placenta of the monkey (Macaca mulatta). 23:23-34, 1965.
Wasmoen,
T.L., Benirschke, K. and Gleich, G.J.: Demonstration of immunoreactive
eosinophil granule protein major basic protein in the placenta and placentae
of non-human primates. Placenta 8:283-292, 1987.
Wasmoen,
T.L., McKean, D.J., Benirschke, K., Coulam, C.B. and Gleich, G.J.: Evidence
of eosinophil granule major basic protein in human placenta. J. Exp. Med.
170:2051-2063, 1989.
Weiss,
G., Weick, F., Knobil, E., Wolman, S.R. and Gorstein, F.: An X-O anomaly
and ovarian dysgenesis in a rhesus monkey. Folia primatol. 19:24, 1973.
Wiener,
A.S., Moor-Jankowski, J. and Gordon, E.B.: Blood groups of apes and monkeys.
V. Studies on the human blood group factors A,B,H and Le in old and new
world monkeys. Amer. J. Phys. Anthropol. 22:175-188, 1964.
Wilken,
J.A., Matsumoto, K., Laughlin, L.S., Lasley, B.L. and Bedows, E.: A comparison
of chorionic gonadotropin expression in human and macaque (Macaca fascicularis).
In press, 2001.
Wilson,
J. G.: Abnormalities of intrauterine development in non-human primates.
Pp. 261-292. In, Symposium on the Use of Non-human Primates for Research
on Problems of Human Reproduction. Sukhumi, 1971. WHO Research & Training
Centre on Human Reproduction, Karolinska, Sweden, 1972.
Wislocki,
G.B. and Streeter, G.L.: On the placentation of the macaque (Macaca
mulatta) from the time of implantation until the formation of the
definitive placenta. Contrib. Embryol. Carnegie Inst. 27:1-66, 1938.
Yamamoto, E., Ino, K. Yamamoto, T., Sumigama, S., Nawa, A., Nomura, S. and
Kikkawa, F: A pure nongestational choriocarcinoma of the ovary diagnosed with short tandem repeat analysis: case report and review of the literature. Int. J. Gynecol. Cancer 17:254-258, 2007.
|