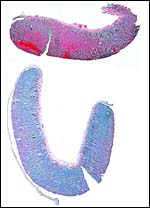| (Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.) |
| Last updated: July 5, 2004. |
Tragelaphus imberbis
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
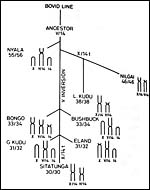
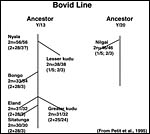
The lesser kudu of Africa is more gracile and considerably smaller than the greater kudu ( Tragelaphus strepsiceros ), weighing 60-100 kg (Nowak, 1999). Its habitat, as that of most “Waldantilopen”, is dense vegetation. They hide during day time and browse in the late evening and early morning. It has remarkable jumping ability. Longevity is over 20 years in Zoological Gardens according to Nowak, but Jones (1993) gave it as being 16 years 11 months. Its name derives thus: Tragelaphus (from Gr. Tragos = he-goat, and elaphos = deer) and imberbis (from L. = beardless) (Gotch, 1979). While twins have been described very rarely, twinning is truly uncommon in lesser kudus. Relatively few Zoological Gardens exhibit this shy and beautiful species from East Africa . Their evolution is highly speculative in the absence of many fossils. Wallace (1976) wrote about the possible relationship of Tragelaphinae and we have further extended these findings. They are considered in some detail and the putative pedigree is shown as the last two pictures in this chapter (Benirschke et al., 1980; Petit et al., 1994/5). Matthee & Robinson (1999) most recently studied the details of mtDNA cytochrome b evolution in this and other groups of Bovidae, but their “tree” looks quite a bit different than the ones derived from chromosome studies.
 |
Family of lesser kudu at Basel Zoo , Switzerland. |
2) General Gestational Data
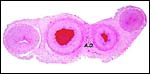
Single young are born after a gestation of 222 days (Dittrich, 1972). Puschmann (1989) lists the gestational length as averaging 254 days, with birth weigh of 6.1 kg. Hayssen et al. (1993) referred to neonatal weights as being 5-6 kg. I had one specimen available, a stillborn male weighing 3,200 g. It was assumed to have succumbed near term or at term from delivery that was complicated by dystocia. It measured 44 cm CR and is depicted below.
 |
Male stillborn, term lesser kudu. |
3) Implantation
Implantation is mesometrial with the cord also being mesometrial. Both uterine horns are used. The placenta is multicotyledonary as in most artiodactyla and has essentially similar features as other species in this order.
4) General Characterization of the Placenta
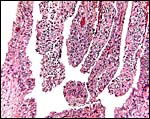
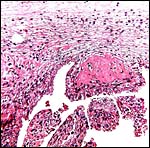
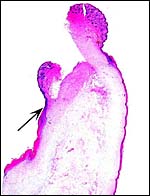
The multicotyledonary placenta weighed 450 g, had 48 cotyledons and these varied in size from 2 to 7 cm in diameter and 0.8 cm in thickness. The greatest width of the placenta was 54 cm. A majority of cotyledons was much congested. This is an epitheliochorial placenta.
 |
Lesser kudu placenta with 48 cotyledons. Congestion of fetal vessels at right. |
5) Details of fetal/maternal barrier
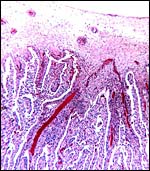
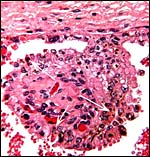
The trophoblast is cuboidal to columnar, especially beneath the chorion. It has numerous binucleate trophoblastic cells and these are even present in the intercotyledonary membranes. The nature and products of binuclear cells is discussed in many other chapters of artiodactyl species (see also Wooding, 1982). Atkinson et al. (1993) defined their glycoprotein products in some detail. Areolae have been described for greater kudu and are presumably present here as well. Beneath the chorion a few villi showed degenerative changes (hyalinization) and some small regions had yellow pigmentation (allegedly the “hematophagous organ”). Iron stains of the pigment were negative. Although no implanted placenta was available, I assume that there is no trophoblastic invasion of the uterus or caruncles.
6) Umbilical cord
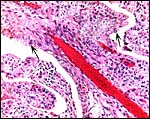
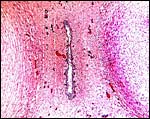
The mesometrially inserted cord was 14 cm long and 2 cm in diameter. It was not spiraled. It contained 4 vessels and a central allantoic duct. On its amnionic portion it was covered with delicate foci of yellowish squamous metaplasia. Near the insertion on the fetus' abdomen a constriction was obvious It is the site were skin and cord meet and although possible, I doubt that it is the site of normal rupture. The allantoic duct has transitional epithelium. Numerous small blood vessels were also present within the cord, as is common of most artiodactyls. A single squamous epithelium covers the cord with tiny foci of keratinization.
7) Uterine circulation
No studies have been conducted.
8) Extraplacental membranes
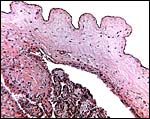
The amnion is a single-layered membrane without blood vessels and showing numerous pronounced foci of keratinization, especially towards the cord insertion. The allantoic epithelium is cuboidal and the allantoic connective tissue is vascularized.
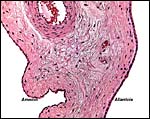 |
The allanto-amnion has blood vessels in the allantoic portion only. |
9) Trophoblast external to barrier
No such infiltration exists.
10) Endometrium
Since I have had no uterus available, the nature of the endometrium is unknown to me and it has not been described.
11) Various features
There is neither subplacenta nor metrial glands.
12) Endocrinology
I am not aware of any endocrine studies in this species.
13) Genetics
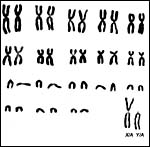
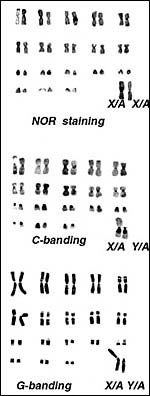
The lesser kudu has 38 chromosomes and is remarkable because of its fusion chromosome; both X and Y chromosomes have compound fusions, and we believe they are with the same autosome (# 13). Petit et al. (1994/5) also studied the relationships of these unusual animals. Both views are presented at the end in a “pedigree” of possible developments over time. The finding of translocations in both sex chromosomes contrasts with that of many other African ungulates that have only an X/A translocation, but possess a normal Y chromosome. These species thus have different numbers in males and females (Benirschke et al., 1980). Another view of evolutionary relationships, resulting from mtDNA study, was presented by Matthee & Robinson (1999).
A hybrid with a female sitatunga was described from the Rome Zoo (Anonymous, 1969), the same report as in Gray (1972). Its fertility is unknown.
14) Immunology
I am not aware of any immunological studies in lesser kudu.
15) Pathological features
Multifocal non-suppurative encephalitis due to Neospora canium infection was diagnosed in term twin lesser kudu and a subsequent singleton from the Hannover Zoo (Peters et al., 2001). During an epidemic of rinderpest, lesser kudu were found to be highly susceptible (Kock et al., 1999). Griner (1983) found dental malocclusion secondary to periodontitis as cause of death in two animals.
Freemartinism of a female as a result of having been born as twin to a male was reported by Robertia (2001).
16) Physiologic data
I am not aware that any studies have been undertaken. Hradecky (1982) studied
the uterine morphology of various African antelopes, including the lesser kudu and stated that it is like that of cattle, “uterus bicornis”.
17) Other resources
Cell strains of this and several other animals as well as related tragelaphines are available from CRES by contacting Dr. Oliver Ryder at: oryder@ucsd.edu .
18) Other remarks – What additional Information is needed?
No early stages of gestation are available and no pregnant uterus has been observed. This is probably the first description, and much more structural information is needed. Also, endocrine studies are absent. It would also be helpful if the putative translocations of chromosomes could be examined using FISH.
Acknowledgement
I appreciate very much the help of the pathologists at the San Diego Zoo.
References
Anonymous: Male lesser kudu x sitatunga hybrid, Rome Zoo. In, Intern. Zoo Yearb., J. Lucas, ed. 9:239, 1969.
Atkinson, Y.H., Gogolin-Ewens, K.J., Hounsell, E.F., Davies, M.J., Brandon , M.R. and Seamark, R.F.: Characterization of placentation-specific binucleate cell glycoproteins possessing a novel carbohydrate. J. Biol. Chem. 268:26679-26685, 1993.
Benirschke, K., Ruedi, D., Muller, H., Kumamoto , A.T. Wagner, K.L. and Downes, H.S.: The unusual karyotype of the lesser kudu, Tragelaphus imberbis. Cytogenet. Cell Genet. 26:85-92, 1980.
Dittrich,, L.: Gestation periods and age of sexual maturity of some African antelopes. Intern. Zoo Yearb. 12:184-187, 1972.
Gotch, A.F.: Mammals – Their Latin Names Explained. Blandford Press, Poole , Dorset , 1979.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2 nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough , England , 1972.
Griner, L.A. : Pathology of Zoo Animals. Zoological Society of San Diego , San Diego , California , 1983.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell University Press, Ithaca , 1993.
Hradecky, P.: Uterine morphology in some African antelopes. J. Zoo An. Med. 13:132-136, 1982.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearb. 32:159-169, 1993.
Kock, R.A., Wambura, J.M., Mwanzia, J., Wamwayi, H., Ndungu, E.K., Barrett, T., Kock , N.D. and Rossiter, P.B.: Rinderpest epidemic in wild ruminants in Kenya 1993-1997. Vet. Rec. 145:275-283, 1999.
Matthee , C.A. and Robinson, T.J.: Cytochrome b phylogeny of the family Bovidae: Resolution within the Alcelaphini, Antilopini, Neotragini, and Tragelaphini. Mol. Phylogenet. Evol. 12:31-46, 1999.
Nowak, R.M.: Walker 's Mammals of the World. 6 th ed. The Johns Hopkins Press, Baltimore, 1999.
Peters, M., Wohlsein, P., Knieriem, A. and Schares, G.: Neospora canium infection associated with stillbirths in captive antelopes ( Tragelaphus imberbis ). Vet. Parasitol. 22:153-157, 2001.
Petit, P., Vermeesch, J.R., Marynen, P. and de Meurichy, W.: Comparative cytogenetic study in the subfamily Tragelaphinae. Proc. 11 th Europ. Coll. Cytogenet. Domest. Anim. Pp. 109-113, 1994/5.
Puschmann, W.: Zootierhaltung. Vol. 2, Säugetiere. VEB Deutscher Landwirtschaftsverlag Berlin , 1989.
Robertia, J.: Freemartinism in lesser kudu. Communiqué, October, p.40, 2001. (This is the official Journal of the AZA [American Zoological Association]).
Wallace, C.: Cytogenetic investigations of certain free-roaming wild mammals in the Kruger National Park . Ph. D. Thesis, p.300. University of Witwatersrand , Johannesberg, 1976.
Wooding, F.B.: The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 31:31-39, 1982.