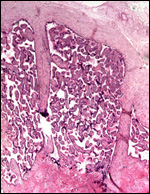
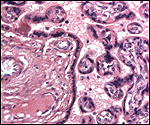
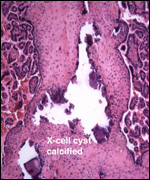
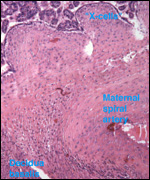
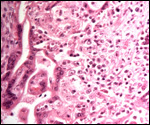
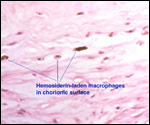
|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
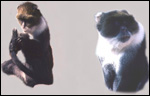
Cercopithecus mitis (kolbi)
Order: Primates
Family: Cercopithecidae
1) General Zoological Data
Of catarrhine African monkeys, the guenons represent a very varied group of primates. They differ much in size, coloration, distribution and cytogenetic characters. Several groups are formally segregated, and the phylogenetic relations are incompletely worked out. Moreover, the taxonomic classification is confusing and has often been revised. The Kolb's monkey is one of these. It is a rarely discussed representative of this group. The Eastern African primate has, therefore, many synonyms. One of these is the "blue monkey", a commonly used designation. The scientific literature now groups Kolb's monkey under Sykes' monkey, or C. mitis (kolbi), which includes, among other less familiar names, the following: C. kolbi, C. albogularis, C. diadematus, C. m. stuhlmanni (Wilson & Reeder, 1992). Others, like Nowak (1999), separate some of these animals into different species with their own denominations, but they may not even list Kolb's monkey. A preliminary, modern phylogeny of guenons was presented only in abstract form by Raaum (2000).
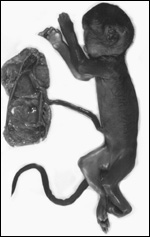
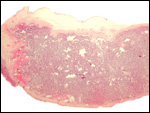
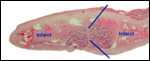
The animals whose placentas are shown here come from the collection of the Zoological Society of San Diego, where reproduction was achieved in the past with Kolb's monkeys. This chapter is based on those pregnancies but also makes reference to some related forms. In addition, I draw on three other placentas of related animal species: the Talapoin money (Miopithecus talapoin), Diana monkey (Cercopithecus diana), Mustached monkey (Cercopithecus cephus), and Mandrill (Mandrillus sphinx).
It should be noted, however, that a separate chapter is devoted to the lesser spot-nosed guenon (Cercopithecus petauritus).
2) General Gestational Data
"Blue monkeys" live in troops in Eastern Africa, have many color
variations and a wide distribution throughout those forests. They also
vary considerably in size. Guenons breed throughout the year and have
birth intervals of about 6 months. Animals observed in the wild by Cords
& Rowell (1987), however, had intervals of between 24 and 54 months.
Gestation is about 5.6 months long, and females produce their first young
when they are about 4-5 years old. Longevity is around 30+ years in captivity.
Many species of guenons (in German: "Meerkatzen") are widely
represented in zoological gardens. The origin of the designation "guenon"
is unknown. Puschmann (1989) has provided much detail concerning their
management in zoos, and provided this specifically for a variety of named
species. Cycles average 30 days, gestational length is about 160-170 days,
and newborns weigh around 400-500 g, depending on the species. In a review
of the twinning frequencies, Geissmann (1990) concluded that the sample
size studied for most species is too small for definitive conclusions
to be reached. He reviewed all reports and concluded that the twinning
frequency is probably lower in Old World Monkeys that is that of humans
(which is around 1:80 births).