| (Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.) |
| Last updated: Feb 7, 2008. |
Eastern
Kiang
Equus kiang
Order: Perissodactyla
Family: Equidae
1) General Zoological Data
Kiangs are described as being largest of the wild asses or hemiones (Nowak,
1999) and are at home in the Tibetan plateau. The name is of Tibetan origin
(Gotch, 1979). They are related to onager and kulan, but have different
chromosome numbers. Large herds have been reported from Tibet in the past
but, more recently, their number has declined. They are not commonly seen
in Zoological Gardens and have never been tamed. Three indistinct subspecies
have been described; the specimen studied here is Equus kiang holderi.
More information and references on evolution and the problem of species
designation in hemiones are found in Ryder & Chemnick (1990). These
are very hardy, tough animals and are noted for the accumulation of fat
during summer seasons. A successful breeding colony exists at San Diego's
Wild Animal Park. The large animals have a bight re-brown upper body and
may weigh up to 400 kg and may live to be 26 years old (Jones, 1993). The
term Equus hemionus kiang is often used in the literature, denoting
its relation to the other hemiones. Krumbiegel (1958) discussed their origin
and relation to the other equidae. Another comprehensive review is by Groves
(1974).
Dolan (1999) has provided a comprehensive overview of the kiangs held in captivity, in Europe as well as North America.
 |
Kiang dam and offspring at San Diego Wild Animal Park. |
 |
Group of kiang at San Diego Wild Animal Park. |
2)
General Gestational Data
A single young is born after a nearly year-long gestation (7-10 months according
to the review by Hayssen et al., 1993). Horses, Equus caballus, have
generally a length of gestation of between 329-345 days, depending on the "breed". This placenta came from the breeding group at San Diego
Zoo's Wild Animal Park and from a dam that had eight previous young. The
neonate was healthy and survived. We have had two neonates die to determine
weights: a male that weighed 36.5 kg and a female with a weight of 29.0
kg. In October 2007 another female delivered a healthy calf; its placenta weighed 2,200 g and the umbilical cord had three normal vessels.
3)
Implantation
Early stages of placentation have not been described, thus nothing is
known of the notorious placental (chorionic) "girdle" (of horses)
in this equine species. Implantation of the placenta in the horse - and
it is probably reasonable to draw this analogy - is relatively late, with
the blastocyst floating and sending signals to the endometrial surface
for days, whilst being shuttled back and forth in the bicornuate uterus.
Equidae have a "copious amount of uterine milk" (Amoroso, 1962;
Ramsey, 1982) in which the blastocyst can be shuttled for some days before
actual implantation. The trophoblast absorbs the nutrients from these
uterine secretions. They are composed of actual secretions and of endometrial
debris from degenerating epithelium that is later restored. Equine implantation,
and most relevant other aspects of placentation of the horse can be found
by Allen & Stewart (2001).
4)
General Characterization of the Placenta
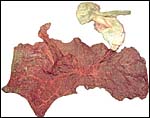
The placenta shown here weighed 3,450 g, and it was 100 cm long and maximally
60 cm in width. It was uniformly thin, about 0.2 cm. The cord was attached
near the center, the membranes had everted. Another placenta received
a few days later weighed 3,700 g, had similar measurements but the umbilical
cord was more completely present. Its allantoic portion was 37 cm long;
the amnionic portion was 30 cm long (67 cm altogether). A placenta that
had been collected earlier came from another dam and weighed only 1,500
g. Its cord, however, measured 44 cm in length and the whole specimen
measured 110 x 103 cm. Yet another placenta from a weak male newborn foal
weighed 2,025 g, measured 120x70 cm and had a 41 cm cord with four vessels,
many small vessels and allantoic duct. Much meconium (400 g) was attached
to the maternal aspect of that placenta, apparently from some neonatal
distress. In September 2003 another placenta became available. It weighed
3,000 g, had a three vessel cord of 84 cm length and marked spiraling.
At the amnionic portion was a "nipple"-like protrusion from
which a hair emanated. It is shown below.
5)
Details of fetal/maternal barrier
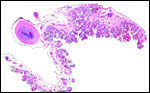
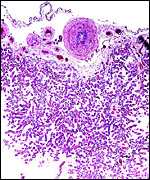
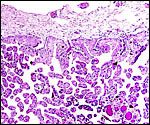
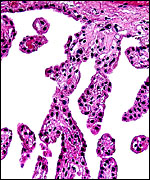
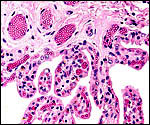
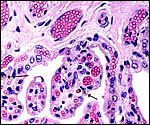
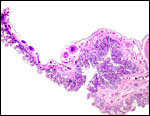
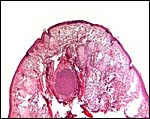
As the other members of the family Equidae, kiangs have a diffuse, villous
epithelio-chorial placenta. The entire surface of the placenta is covered
with short, slightly branched villi that are covered by a single layer of
trophoblast. Binucleate cells, as found in ruminants, are not present. Beneath
the chorionic surface and in the short intervillous stretches especially,
the trophoblast is mostly cylindrical, has short microvilli and often some
yellow pigmentary inclusions. Amoroso (1961) mentioned fat droplets in the
cytoplasm.
I assume that this placenta, because of the ability of kiangs to hybridize
with several other equids, is very similar to that described for the horse.
Then, the statement by Ramsey (1982) may be true that, initially, there
is only "flat" contact with the endometrium, and villi do not
form (to interdigitate with maternal endometrium) until the seventh to eight
weeks of gestation. On the other hand, no large stretches of "smooth
chorion" are found between the villi as she described. True, there
are small stretches, but the low power views that follow here show
that there is a pretty uniform distribution of villi. Certainly, there were
nothing like "miniature cotyledons", to which Amoroso (1961) made
reference. I have sampled many different regions of these placentas and
they are all similar, without large stretches of smooth chorion.
6)
Umbilical cord
The umbilical cord of the first placenta was 25 cm long and 1.8 cm thick.
It contained three blood vessels. There was moderate left-directed spiraling.
It is highly likely that the umbilical cord of this specimen was incomplete.
This is so because 1) the cords of the other placentas were so much longer
(67 and 44 cm); 2) because horse umbilical cords vary between 50 and 100
cm; 3) the membranes usually attached midway between fetus and placental
surface (where the allantoic and amnionic cavities meet) and no cord was
found distal to that attachment in the first placenta but a 30 cm segment
was in the second. The thickness and firmness of the blood vessels was remarkable.
They were very difficult to section. Numerous somewhat smaller blood vessels
are present next to the large vessels. The placenta that had been collected
earlier, while lighter, had a longer cord. It measured 44 x 1.5 cm. The
surface has no verrucal protrusions, contrary to those reported to exist
in horses (Amoroso, 1961; Ramsey, 1982). It should be noted, however, that
this is true only of the allantoic portion of cord. There was extensive
squamous metaplasia of the amniotic portion of the second placenta's cord.
In the second to last-collected placenta, the cord had four large vessels in addition to numerous small vessels and the allantoic duct.
The most recent placenta had 3 vessels, a "nipple" on the amnionic
portion with fine vasculature and the cord was 84 cm long and heavily spiraled.
In the very central portion of the cord are small remnants of dark cylindrical
cells without forming a true duct. They may be the remains of the vitelline
structure that, earlier in gestation, played a significant role in horse
placentation.
Since the original description we have had a newborn (that died immediately) in whom the long umbilical cord was wrapped around the hind leg two and one half times. It is shown below.
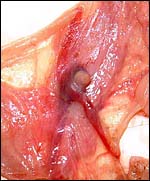
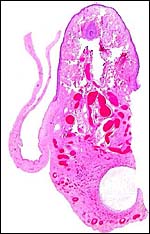
A remarkable aspect of this last placenta was the presence, in a piece of membrane that was attached to the cord, of what can best we described as a "dermoid", or a benign teratoma. It had normal skin, pieces of cartilage, fat and a cyst with cylindrical, mucus-producing epithelium. The next two pictures show this same "dermoid". It may be of similar origin as the teratoma we found in a horse placenta (Gurfield & Benirschke, 2003). Another lesion like this had been seen but was misinterpreted two years earlier. Now, in the third and last-collected placenta we observed a nipple-like structure whose origin may be the same. It is shown below as well.
7) Uteroplacental circulation
There are certainly no studies in kiangs, and for an access to the horse
fetal physiology, the reader is directed to the review by Hayssen et al.
(1993) which provides ample references.
8)
Extraplacental membranes
The vascularized allantoic sac of equines is huge and surrounds the relatively
smaller amnionic cavity. Indeed, the amnion is closely approximated to
the fetus' surface in early gestation. Soft yellow hippomanes were present
in this specimen only in the allantoic sac; others have suggested that
it is also found in the amnionic cavity.
 |
The apposing allantoic (left) and amnionic membranes (right). |
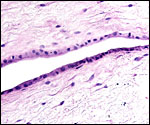 |
The amnion is avascular and covered by a thin squamous epithelium Verrucae were not found in the kiang. |
9)
Trophoblast external to barrier
There is no invasion of trophoblast into the endometrium or fusion with
endometrial cells.
10)
Endometrium
The equine placenta is a "non-deciduate" placenta (Amoroso,
1961). Typical decidua is said to form only in the region of the girdle,
the "endometrial cup" as this region of the pregnant horn was
once called. If this is the case in the kiang, that is as yet unknown,
but it is a significant feature of horse placentas. The remainder of the
endometrium is glandular throughout gestation and secretes fluid that
is absorbed, especially in the "areolae", regions between villous
stems. The epithelium of the endometrium is in close proximity to the
trophoblast and both epithelia show protrusions of capillaries.
11)
Various features
There are no other known specific features.
12)
Endocrinology
The most remarkable feature of horse gestation is the presence of the
temporary chorionic girdle. It is beautifully illustrated by Enders et
al. (1996). This tissue produces the equine chorionic gonadotropin and
has been studied in horses, donkeys and their reciprocal hybrids. Whether
a similar structure or, for that matter the hormone, exists in the kiang
has not been ascertained.
Kiang fetuses have the same large gonads as found in the horse and other
equidae. Their hormonal output has not been studied but is well known
for the horse (see Allen & Stewart, 2001).
13)
Genetics
Ryder & Chemnick (1990) performed the only chromosome analysis of the
Tibetan wild ass, Equus kiang. They found animals with a polymorphism of
## 51 and 52 chromosomes and suggested that the Robertsonian fusion/fission
mechanism of two chromosomes operated that produced the different chromosome
numbers of the related kulan and onager (54-56 elements - see Ryder, 1978).
From mtDNA fragments study (1% difference against kulan and onager), they
suggested that the split between these taxa occurred less than 500,000 years
ago. Additional considerations of the Robertsonian system in asses and hemiones
may be found in Houck et al. (1998).
A variety of hybrids have been reported but little is known of their fertility.
Gray (1972) reported hybrids with horse, donkeys, onager, khur and Burchell
zebra.
 |
Male Kiang with 2n=52 and separate ## 22/23 chromosomes. |
 |
Male Kiang karyotype with 2n=51 and fusion of 22/23 chromosomes. |
14)
Immunology
Much work has been done in exploring the T-cell-defined alloantigens in
horse, donkey and their hybrids (see for instance Baker et al., 2001), but
no similar work has been reported on hemiones, so far as I can determine.
More likely than not, similar systems exist in this species.
15)
Pathological features
Nothing has been written about kiang pathology. In our experience, trauma
was the cause of most death and a neonate died from omphalitis. The last
placenta obtained contained a typical teratoma, a "dermoid",
in its membranes that were adjacent to the mid-portion of the umbilical
cord. What is interesting is that this occurred with a male foal, as the
usual "dermoids" known are those derived from ovaries. One can
only speculate that this lesion did not derive from a germ cell but perhaps
from a stem cell. Its location precludes, I believe, the presence of an
aborted twin. Barr bodies could not be found in the lesion's cells. Aside
from these observations, the foal was weak after birth, had to be hand-raised
and had a thick, "meaty" umbilical stump.
Neonatal death with entanglement of the cord is described above.
16) Physiologic data
There has been an enormous amount of work on the physiology of the horse,
especially the reproductive biology. I have been unable, however, to ascertain
similar references for any hemione. The horse data can be accessed by
perusing the biographical references listed in Hayssen et al. (1993).
17)
Other resources
Numerous cell strains of this and related equids are available from CRES
at San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other remarks - What additional Information is needed?
Early stages of the placenta need to be studied, especially in order to
elucidate whether a chorionic girdle exists. Likewise, endocrine studies
are needed.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Allen, W.R. and Stewart, F.: Equine placentation. Reprod. Fertil Dev.
13:623-634, 2001.
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall's Physiology of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown & Co. Boston, 1961.
Baker,
J.M., Stidworthy, M., Gull, T., Novak, J., Miller, J.M. and Antczak, D.F.:
Conservation of recognition of antibody and T-cell-defined alloantigens
between species of equids. Reprod. Fertil. 13:635-645, 2001.
Dolan, J.M. Jr.: An overview of the kiangs, Equus kiang Moorcroft, 1841, kept in European and North American Zoological collections prior to 1970. Equus 2 (#3)253-268, 1999. Tierpark Berlin .
Enders, A.C., Meadows, S., Stewart, F. and Allen, W.R.: Failure of endometrial cup development in the donkey-in-horse model of equine abortion. J. Anat. 188:575-589, 1996.
Houck, M.L., Kumamoto, A.T., Cabrera, R.M. and Benirschke, K.: Chromosomal rearrangements in a Somali wild ass pedigree, Equus africanus somaliensis (Perissodactyla, Equidae). Cytogenet. Cell Genet. 80:117-122, 1998.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979
Gray,
A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition.
Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Groves, C.P.: Horses, Asses and Zebras in the Wild. Ralph Curtis Books,
Hollywood, Florida, 1974.
Gurfield, N. and Benirschke, K.: Teratoma of the placenta in a horse.
Vet. Path. 40:585-588, 2003.
Hayssen, V., van Tienhoven, A. and van Tienhoven, A.: Asdell's Patterns
of Mammalian Reproduction: a Compendium of Species-specific Data. Comstock/Cornell
University Press, Ithaca, 1993.
Krumbiegel, I.: Einhufer. A. Ziemsen Verlag , Wittenberg, 1958.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Ramsey, E.M.: The Placenta. Human and Animal. Praeger Publ. NY, 1982.
Ryder, O.A.: Chromosomal polymorphism in Equus hemionus. Cytogenet. Cell Genet. 21:177-183, 1978.
Ryder, O.A. and Chemnick, L.G.: Chromosomal and molecular evolution in Asiatic wild asses. Genetica 83:67-72, 1990.