|
(Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.)
|
Aepyceros melampus
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
The impala is a common and fairly widely distributed African ungulate with several subspecies. Commonest in East Africa is Aepyceros melampus rendilis, while A. m. petersi, the black-faced impala, is the most endangered subspecies and distributed further South. Other nominated subspecies are less well known and controversy as to their designation still exists. Impalas are frequently kept as a game animal on ranches in South Africa. The name "Aepyceros melampus" derives from aipos (Greek = high, lofty), keras (horn - because of the long lyre-shaped horns of males), melas (black) and pous (foot). Impala is a Zulu name (Gotch1979).
The precise origin of the impala has remained uncertain. Vrba & Schaller (2000)
suggested that the Aepycerotini split off the caprine taxa approximately 6.5 MYA. Gatesy et al. (1997) attempted to resolve its origin with data from ribosomal DNA studies but were unable to do so. Likewise, Matthee & Davis (2001) were also unable to classify the "enigmatic taxa" impala, suni and klipspringer by their study of nuclear DNA. They suggested a contemporaneous origin in Africa. Another study, of mtDNA, was undertaken in kudu and impala by Nersting & Arctander (2001) that failed to resolve the issue completely as well. They suggested that the more vulnerable black-faced impala may have "hybridized" with the "common" impala (Aepyceros m. melampus). The karyotype of 2n=60 with only acrocentrics may indicate a rather "primitive" antelope (see section on Genetics, below). On the basis of its cerebral and dental structures, Thenius (1969) felt that impala should not be grouped with the gazelles.
Adult female impalas weigh 40-45 kg; males are 60-65 kg. Neonates weigh around 5 kg. Females have four nipples.
 |
Kenya impala (Aepyceros melampus rendilis) at San Diego Wild Animal Park. |
The gestational length of impala pregnancies as ascertained by several authors has been given in the review of Mentis (1972). It has been recorded as being "6½ -7 months; 150-180 days, 171 days, 180-210 days; 196 days, 194,196,197 and 200 days". The species has been described as being monotocous, but several twins have occurred, and one set of twins was associated with a single corpus luteum, suggesting MZ twinning. The longevity record for a black-faced impala is 17 years and 9 months (Jones, 1993).
Roettcher et al. (1970) studied the skulls of 100 postnatal impalas of known ages and presented data that allows age determination. At age 2½ years, maturity is attained, even though estrus may occur before then. In the same publication, the authors presented the CR-lengths and weights (and some other characteristics) of 30 fetal impala specimens. They then constructed a graph that, with these determinations, should allow one to date the gestational age of the growing fetus, assuming the gestation to be approximately 190 days long.
3)
Implantation
Only the right uterine horn is used in impala gestations (Lee et al.,
1977), although both ovaries ovulate as can be seen from the table of
implantations given by Kayanja & Epelu-Opio (1976). The cord attaches
mesometrially (Lee et al., 1977; Mossman, 1987). No descriptions of early
stages of implantation have been published, and I have none available
myself.
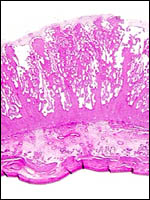
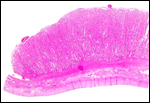
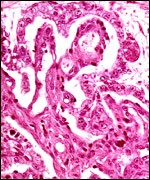
4) General Characterization of the Placenta
The impala has a polycotyledonary placenta with the cotyledons arranged
in four rows and measuring up to 4 cm in greatest diameter. There are
around 50 cotyledons. The best histological pictures available to me were
kindly made available by Dr. Petr. Hradecky (1983).
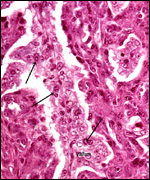
An extensive description of the electron microscopic details of impala placentas from different early stages (fetal CR measurements from 0.8 to 55 cm) of implantation has been published by Kayanja & Epelu-Opio (1976). From this, the authors concluded this to be a typical epithelio-chorial type of placentation with "interlocking of microvilli from trophoblast and maternal epithelium". The trophoblastic epithelium is cuboidal with a microvillous surface. Relatively small numbers of the typical ruminant binucleate cells were present in my preparations. The binucleate cells, thought to produce placental lactogen and perhaps other glycoproteins, are much larger and darker than the cuboidal trophoblast. The microvillous trophoblastic surface abuts the flattened (and occasionally absent) maternal endometrial surface epithelium. The nuclei of the latter are dark and condensed, much different from the loosely-structured trophoblast. The fine structural study disclosed that many epithelial cells were degenerating and that some of this debris was removed by trophoblast. These authors doubted, however, that phagocytosis alone was able to remove all of the debris. Invasion of endometrium or myometrium has not been seen. No pigment of the cylindrical subchorionic trophoblast was present in my sections and there were no degenerative changes in the apices of maternal septa, nor was there hemorrhage. The trophoblast of the membranes between cotyledons is very tall and cylindrical. The villi have very sparse connective tissue and rare Hofbauer cells.
The umbilical cord attaches mesometrially, halfway along the uterine horn (Lee et al., 1977; Mossman, 1987). One umbilical cord measured 17 cm and had no spirals. It contained 4 large blood vessels and an allantoic duct. In addition, numerous mall blood vessels were present.
7)
Uteroplacental circulation
There have been no studies to my knowledge.
8)
Extraplacental membranes
The amnion is delicate, lacks blood vessels and, in my preparations, it
did not have regions of squamous metaplasia although Mossman (1987) listed
"callosities". The allantoic sac is large and tubular. Its membranes
have a rich supply of small blood vessels. Hippomanes were not found.
The epithelium is cuboidal to mostly cylindrical.
9) Trophoblast external to barrier
There is no trophoblastic infiltration into the endometrium or blood vessels.
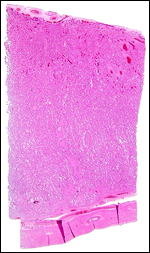
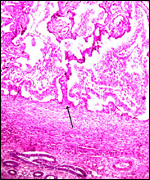
10) Endometrium
The uterus is bicornuate and was described by Hradecky (1982). The caruncles
are arranged in four rows, best seen in the uteri of neonates. Their stroma
contains a number of stout, very muscular arteries and rather fewer glands
than the regions between caruncles. Following gestation and subsequent
to complete uterine involution, hemosiderin-laden macrophages are present
in the stroma. During their fine-structural study of impala cotyledons,
Kayanja & Epelu-Opio (1976) showed that there is continued secretion
by the endometrial epithelium, although it lessened toward term. The caruncles
of non-pregnant endometrium are relatively fibrous, with most of the glands
deeply situated, compared with their more superficial location in the
endometrium between the caruncles. The epithelium is cuboidal but, in
the crypts of the cotyledons, the epithelium is very flat and often inapparent
or absent. Decidualization as seen in primates is absent. One of my uteri
had collections of lymphocytes and plasma cells, in addition to the hemosiderin
deposits. Perhaps this is part of an involutionary process.
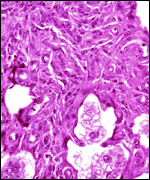
 |
Two maternal endometrial caruncles in a nonpregnant uterus. |
There are no other noteworthy features.
12) Endocrinology
Millar & Aehnelt (1977) developed a sensitive antibody-based radioimmunoassay
for the measurement of LH that correlated well with the reproductive state
of impala and a wide variety of other African species. Illius et al. (1983)
found that the testosterone levels of territorial males were higher than
those of bachelors following a challenge with LH-RH. Basal LH and FSH
levels were similar in seasons, but there was a marked difference in other
parameters studied by Brown et al. (1991). Thus GnRH and hCG responses
with testosterone output and increased testicular size were much greater
during rutting season, and the standardized electroejaculations showed
much better semen in the rutting season. Gilmore et al. (1998) provided
data and protocols for the successful conservation of semen in impala
and several other species.
A preliminary assessment of the number of prolactin-immunoreactive pituitary
cells was undertaken by v.d. Merwe et al. (1999) in lactating and nonlactating
impala ewes. There was the expected increase in prolactin secreting cells
during lactation but a correlation with stress proved impossible.
Schoeman et al. (1998) stained the intestinal mucosa of impala for peptide-containing
cells (serotonin, somatostatin, gastrin, cholcystokinin, glucagons, neurotensin,
secretin, motilin, etc.). They found the distribution of these cells to
be more comparable to that found in sheep than in other game herbivores.
Neonatal testes of my collection had an abundance of interstitial cells
but they were only minimally stimulated. A neonatal ovary showed several
Graafian follicles developing.
13) Genetics
Impalas of all subspecies studied so far have 60 chromosomes, all of which
are acrocentrics, except the small, metacentric Y chromosome (Wurster
& Benirschke, 1968). Wallace and Fairall (1967), however, who studied
numerous animals from the Kruger National Park, found 17animals with 2n=60,
14 with 2n=59, and 3 with 2n=58. The lower numbers were due to fusion
metacentrics and had absolutely no apparent ill-effect upon the animals.
Moreover, numerous fetuses were studied from this population and no congenital
anomalies were identified.
It is my view that much of mammalian speciation results from "Robertsonian
fusion" of acrocentrics to produce metacentric chromosomes, and the
results by Wallace & Fairall (1969) may be such an example. All of
our specimens have had 2n=60 and no impala has had the autosome-X translocation
that is so commonly present in African antelopes and gazelles. From this
observation we have inferred that the ancestral stock that evolved into
the impala was one of the early immigrants into Africa and has remained
separated from those numerous species with this X/A translocation (Effron
et al., 1976).
Grobler & v.d. Bank (1992) presented data on their study of 150 animals
that were founded by combining 33 animals from 7 different localities;
they showed that these animals had retained 90% of their genetic diversity,
when compared to that found in 11,000 other impala. Hybrids with other
species have not been described.
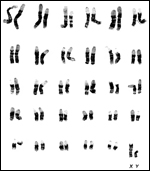 |
Banded karyotype of male black-faced impala (Aepyceros melampus petersi) with 2n=60. |
 |
Karyotype of female Kenyan impala (Aepyceros melampus rendilis). |
I am not aware of any studies.
15)
Pathological features
Knottenbelt (1990) studied the causes of impala mortality in game farms
of Zimbabwe. Stress and injury during capture were the most important
causes of death. Next, infection with Fasciola gigantica was a
significant problem. They stressed the need for adequate nutrition in
winter months. Parasitic infection is another major problem in this species.
In a continuing sampling study of animals from Nature Reserves, Horak
(1978) found infections with Fasciola gigantica, Gongylonema
pulchrum, Haemonchus placei, Longistrongylus sabeii,
and with Impalaia tuberculata. They found an increasing load to
one year of age, when it reached a maximum. Gallivan et al. (1989) found
that 85%+ of impala in Swaziland had infection with Pneumostrongylus
calcaratus (lungworm) but, despite this heavy rate of infection and
the resulting granulomatous lesions, they felt that this did not pose
a significant problem for the animals. Another common infection was described
that affected liver nematodiasis (Gallivan et al., 1996). Du Plessis et
al. (1997a) identified Ehrlichia platys in the blood platelets
of impala, although the animals were apparently healthy. Experimental
infection with the organism causing heartwater (Cowdria ruminantium)
caused no disease (Peter et al., 1999). The effect of ivermectin therapy
in parasitic infections of impala was found to be highly beneficial in
a study published by Horak et al. (1983).
Foot-and-mouth disease occurred in the Kruger National Park in 1992, and
was recorded by Keet et al. (1996). These authors, and Bastos et al. (2000),
identified the viruses recovered from impala and buffalo. Anderson et
al. (1993) found seropositive impala following the epidemic. The virus
responsible for malignant catarrhal fever of ruminants has also been identified
in impalas (Heuschele et al., 1984).
Gummow et al. (1991) showed a correlation of copper toxicity in ruminants
of the Kruger Park with the distance from copper smelters. They shot 26
impala and measured the copper levels and also studied two dead impala,
apparently the result of intoxication. These latter animals showed hepatic
and renal toxicity, and this was presumably the result of hemolysis. Grobler
(1999) showed that the copper concentration of surface soil and plants
was significantly elevated near the copper-smelting plant and thus probably
correlated with copper poisoning of impalas. Subsequently, Grobler &
Swan (1999) attempted to produce copper poisoning in captive impalas but
failed to induce the illness. Ackerman et al. (1999) identified vacuolar
lesions in the spermatozoal neck region that correlated with copper levels
and general body condition.
Griner (198) did necropsies on 88 impala at the San Diego Zoo and found
trauma and sequelae of stress or malnutrition (in young) the most common
causes of death. One uterine adenocarcinoma was also found.
16)
Physiologic data
Platelets were found to be exceedingly small in a study by du Plessis
et al. (1997b). Karesh et al. (1997) examined blood parameters and serologic
titers in black-faced impala of Namibia during a translocation operation.
They found antibodies to bovine rhinotracheitis virus and bovine viral
diarrhea. Pitts & Mitchell (2003) compared the hydrolysis of succinylcholine
in elephants with that in impala and found it to be very significantly
shorter in elephants. Cheney & Hattingh (1988) recommended that xylazine
should be added to etorphine during the immobilization of impala.
17)
Other resources
At present we have cell lines of Kenyan and black-faced impala, all with
2n=60 at CRES
of the San Diego Zoo. They can be made available through contacting Dr.
O. Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional Information is needed?
No early stages of implantation are available and there are no data on
endocrine parameters during gestation.
Acknowledgement
The animal picture was kindly made available by the department of photography
at the San Diego Zoo. Dr. Petr Hradecky kindly made slides available of
freshly fixed material.
References
Ackerman, D.J., Reinecke, A.J., Els, H.J., Grobler, D.G. and Reinecke,
S.A.: Sperm abnormalities associated with high copper levels in impala
(Aepyceros melampus) in the Kruger National Park, South Africa.
Ecotoxicol. Environ. Saf. 43:261-266, 1999.
Anderson, E.C., Foggin, C., Atkinson, M., Sorenson, K.J., Madekurozva, R.L. and Nqindi, J.: The role of wild animals, other than buffalo, in the current epidemiology of foot-and-mouth disease in Zimbabwe. Epidemiol. Infect. 111:559-563, 1993.
Bastos, A.D., Boshoff, C.I., Keet, D.F., Bengis, R.C. and Thomson, G.R.: Natural transmission of foot-and-mouth disease virus between African buffalo (Syncerus caffer) and impala (Aepyceros melampus) in the Kruger National Park, South Africa. Epidemiol. Infect. 124:591-598, 2000.
Brown, J.L., Wildt, D.E., Raath, J.R., de Vos, V., Janssen, D.L., Citino, S.B., Howard, J.G. and Bush, M.: Seasonal variation in pituitary-gonadal function in free-ranging impala (Aepyceros melampus). J. Reprod. Fertil. 93:497-505, 1991.
Cheney, C.S. and Hattingh, J.: Effects of chemical immobilization on the blood composition of impala (Aepyceros melampus (Lichtenstein). J. South Afr. Vet. Assoc. 59:13-18, 1988.
Du Plessis, L., Reyers, F. and Stevens, K.: Morphological evidence for infection of impala, Aepyceros melampus, platelets by rickettsia-like organism. Onderstepoort J. Vet. Res. 64:317-318, 1997a.
Du Plessis, L., Botha, A.J. and Stevens, K.: Impala, Aepyceros melampus, platelets: count, morphology, and morphometric observations. Tissue cell 29:217-220, 1997b.
Effron, M., Bogart, M.H., Kumamoto, A.T. and Benirschke, K.: Chromosome studies in the mammalian subfamily Antilopinae. Genetica 46:419-444, 1976.
Gallivan, G.J., Barker, I.K., Alves, R.M., Culverwell, J. and Girdwood, R.: Observations on the lungworm, Pneumostrongylus calcaratus, in impala (Aepyceros melampus). J. Wildl. Dis. 25:76-82, 1989.
Gallivan, G.J., Barker, I.K., Culverwell, J. and Girdwood, R.: Prevalence of hepatic helminthes and associated pathology in impala (Aepyceros melampus) in Swaziland. J. Wildl. Dis. 32:137-141, 1996.
Gatesy, J., Amato, G., Vrba, E., Schaller, G. and DeSalle, R.: A cladistic analysis of ribosomal DNA from the Bovidae. Mol. Phylogenet. Evol. 7:303-319, 1997.
Gilmore, J.A., McGann, L.E., Ashworth, E., Acker, J.P., Raath, J.P., Bush, M. and Critser, J.K.: Fundamental cryobiology of selected African mammalian spermatozoa and its role in biodiversity preservation through the development of genome resource banking. Anim. Reprod. Sci. 53:277-297, 1998.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Grobler, J.P. and van der Bank, F.H.: Genetic diversity in isolated sable and impala populations. In, The Sable Antelope as a Game Ranch Animal. Pp. 22-25, Proceedings of a Symposium held at Onderstepoort, South Africa, September, 1992.
Grobler, D.G.: Copper poisoning in wild ruminants in the Kruger National Park: geobotanical and environmental investigation. Onderstepoort J. Vet. Res. 66:81-93, 1999.
Grobler, D.G. and Swan, G.E.: Attempted induction of chronic copper poisoning in boma confined impala. Onderstepoort J. Vet. Res. 66:169-174, 1999.
Gummow, B., Botha, C.J., Bassou, A.T. and Batianello, S.S.: Copper toxicity in Ruminants: air pollution as a possible cause. Onderstepoort J. Vet. Res. 58:33-39, 1991.
Heuschele, W.P., Oosterhuis, J., Anderson, M.P., Swansen, M. and Fletcher, H.R.: Malignant catarrhal fever in wild ruminants. Chapter 25 (pp. 296-308) In, One Medicine. O.A. Ryder and M.L. Byrd, eds., Springer-Verlag, Berlin, 1984.
Horak, I.G.: Parasites of domestic and wild animals in South Africa. X. Helminths in impala. Onderstepoort J. Vet. Res. 45:221-228, 1978.
Horak, I.G., Boomker, J., Kingsley, S.A. and de Vos, V.: The efficacy of ivermectin against helminth and arthropod parasites of impala. J. S. Afr. Vet. Assoc. 54:251-253, 1983.
Hradecky, P.: Uterine morphology in some African antelopes. J. Zoo Anim. Med. 13:132-136, 1982.
Hradecky, P.: Placental morphology in African antelopes and giraffes. Theriogenology 20:725-734, 1983.
Illius, A.W., Haynes, N.B., Lamming, G.E., Howles, C.M., Fairall, N. and Millar, R.P.: Evaluation of LH-RH stimulation of testosterone as an index of reproductive status in rams and its application in wild antelope. J. Reprod. Fertil. 68:105-112, 1983.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Karesh, W.B., Rothstein, A., Green, W., Reuter, H.O., Braselton, W.E., Torres, A. and Cook, R.A.: Health evaluation of black-faced impala (Aepyceros melampus petersi) using blood chemistry and serology. J. Zoo Wildl. Med. 28:361-367, 1997.
Kayanja, F.I. and Epelu-Opio, J.: The fine structure of the placenta of the impala Aepyceros melampus (Lichtenstein, 1812). Anat. Anz. 139:396-410, 1976.
Keet, D.F., Hunter, P., Bengis, R.G., Bastos, A. and Thomson, G.R.: The 1992 foot-and-mouth disease epizootic in the Kruger National Park. J. S. Afr. Vet. Assoc. 67:83-87, 1996.
Knottenbelt, M.K.: Causes of mortality in impala (Aepyceros melampus) on 20 game farms in Zimbabwe. Vet. Rec. 127:282-285, 1990.
Lee, S.Y., Mossman, H.W., Mossman, A.S. and del Pino, G.: Evidence of a specific nidation site in ruminants. Amer. J. Anat. 150:631-640, 1977.
Matthee, C.A. and Davis, S.K.: Molecular insights into the evolution of the family Bovidae: a nuclear DNA perspective. Mol. Biol. Evol. 18:1220-1230, 2001.
Mentis, M.T.: A review of some life history features of the large herbivores of Africa. The Lammergeyer 16:1-89, 1972.
Merwe, P.v.d., Meltzer, D.G. and van Aswegen, G.: Influence on the Prolactin secreting cells of the hypophysis of impala (Aepyceros melampus): and immunocytochemical and computer image analysis study. Onderstepoort J. Vet. Res. 66:151-156, 1999.
Millar, R.P. and Aehnelt, C.: Application of ovine luteinizing hormone (LH) radioimmunoassay in the quantitation of LH in different mammalian species. Endocrinology 101:760-768, 1977.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nersting, L.G. and Arctander, P.: Phylogeography and conservation of impala and greater kudu. Mol. Ecol. 10:711-719, 2002.
Peter, T.F., Anderson, E.C., Burridge, M.J., Perry, B.D. and Mahan, S.M.: Susceptibility and carrier status of impala, sable, and tsessebe for Cowdria ruminantium infection (heartwater). J. Parasitol. 85:468-472, 1999.
Pitts, N.I. and Mitchell, G.: In vitro succinylcholine hydrolysis in plasma of the African elephant (Loxodonta Africana) and impala (Aepyceros melampus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 134:123-129, 2003.
Roettcher, D., Hoffmann, R.R. and Kayanja, F.I.B.: Ergebnisse der prae- und postnatalen Altersbestimmung beim ostafrikanischen Impala (Aepyceros melampus Lichtenstein, 1812). Z. Säugetierk. 35:289-305, 1970.
Schoeman, J.H., de Vos, V. and van Aswegen, G.: Distribution of endocrine cells in the gut of the impala (Aepyceros melampus). Onderstepoort J. Vet. Res. 65:31-35, 1998.
Thenius, E.: Stammesgeschichte der Säugetiere (einschließlich der Hominiden). In, Handbuch der Zoologie, J.G. Helmcke, D. Starck and H. Wermuth, eds. 8(2):369-722, 1969.
Vrba, E.S. and Schaller, G.B., eds.: Antelopes, Deer, and Relatives. Fossil Record, Behavioral Ecology, Systematics, and Conservation. Yale University Press, New Haven, 2000.
Wallace, C. and Fairall, N.: Chromosome polymorphism in the impala (Wallace, C. and Fairall, N.: Chromosome polymorphism in the impala (Aepyceros melampus melampus). S. Afr. J. Sci. 63:482-486, 1967.
Wurster, D.H. and Benirschke, K.: Chromosome studies in the superfamily Bovoidea. Chromosoma 25:152-171, 1968.