| |
16)
Physiologic data
A comprehensive review on physiologic data in rock hyraxes was presented
by Rubsamen et al. (1982). McNairn & Fairall (1984) described temperature
adaptations of dassies with respect to climate adaptation. The gastrointestinal
tract of hyraxes, known to be unusual because of the presence of two appendices,
was studied by Bjornhag et al. (1994, 1995). The intestinal tract, including
their double appendices (ceca), was depicted by Griner (1968).
Hyraxes have nondescended testes; they are "testicond". Their
testes are located at the lower renal poles. This anatomic feature was studied
by v.d. Schoot (1996) in the fetal development of rock hyraxes. The findings
suggested that there was only minimal, partial development of the gubernaculum
and the primordia of the cremaster sac were absent.
The study of hyrax brains has disclosed the presence of an unusual, small
structure in the aqueduct (Quay, 1971). This is a small ependymal proliferation
whose function is unknown.
17) Other resources
The "Frozen Zoo" of CRES
at the San Diego Zoo has numerous cell lines of both sexes from rock
hyraxes. They may be made available by contacting Dr. O. Ryder at oryder@ucsd.edu.
18)
Other remarks - What additional information is needed?
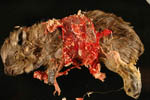
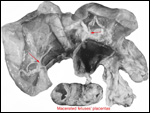
The multiple gestations of hyraxes have always been believed to be multizygotic.
The report of Soma et al. (1976) suggested that MZ littermates may occur.
More correlation with the number of corpora lutea or RFLP study of DNA
differences is warranted. The secretory product of the large "interstitial
gland" of the ovaries needs exploration. There are few good studies
of umbilical cord (length, structure), and placental weights are lacking.
Acknowledgement
I appreciate very much the help of the pathologists at the San Diego Zoo.
References
Assheton, R.: The morphology of the ungulate placenta, particularly the
development of that organ in sheep, and notes upon the placenta of the
elephant and hyrax. Phil. Trans. Roy.Soc. B 198:143-220, 1906.
Bjornhag,
G., Becker, G., Buchholz, C. and von Engelhardt, W.: The gastrointestinal
tract of the rock hyrax (Procavia habessinica). 1. Morphology and
motility patterns of the tract. Comp. Biochem. Physiol. A Physiol. 109:649-653,
1994.
Bjornhag,
G., Becker, G., Heller, R.. and von Engelhardt, W.: The gastrointestinal
tract of the rock hyrax (Procavia habessinica). 2. Fluid flow,
production of short chain fatty acids and absorption of water and e electrolytes.
Comp. Biochem. Physiol. A Physiol. 111:433-438, 1995.
Cousins,
D.V., Peet, R.I., Gaynor, W.T., Williams, S.N. and Gow, B.L.: Tuberculosis
in imported hyrax (Procavia capensis) caused by an unusual variant
belonging to the Mycobacterium tuberculosis complex. Vet. Microbiol.
42:135-145, 1994.
Dempsey,
E.W.: Comparative aspects of the placenta of certain African mammals.
J. Reprod. Fertil. Suppl. 6:189-192, 1969.
Fourie,
P.B.: The life-span of mammals: estimates for the dassie (Procavia
capensis). J. S. Afr. Vet. Assoc. 49:143-145, 1978.
Frye,
F.L.: Iron storage disease (hemosiderosis) in an African rock hyrax ().
J. Zoo Anim. Med. 13:152-156, 1982.
Gerlach,
G. and Hoeck, H.N.: Islands on the plains: metapopulation dynamics and
female biased dispersal in hyraxes (Hyracoidea) in the Serengeti National
Park. Mol. Ecol. 10:2307-2317, 2001.
Glover,
T.D. and Sale, J.B.: The reproductive system of male rock hyrax (Procavia
and Heterohyrax). J. Zool. 156:351, 1968.
Gombe,
S., Oduor-Okelo, D. and Amoroso, E.C.: Role of progesterone in pregnancy
of the hyrax. E. Afr. J. Med. Res. 4:133-134, 1977.
Griner,
L.A.: The rock hyrax (Procavia capensis): a potential laboratory
animal. Lab. Anim. Care 18:144-150, 1968.
Griner,
L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego,
California, 1983.
Heap,
R.B., Gombe, S. and Sale, J.B.: Pregnancy in the hyrax and erythrocyte
metabolism of progesterone. Nature 257:809-811, 1975.
Horak,
I.G. and Fourie, I.J.: Parasites of domestic and wild animals in South
Africa. XIX. Ixodid ticks and fleas on rock dassies Procavia capensis
() in the Mountain-Zebra National Park. Onderstepoort J. Vet. Res. 53:123-126,
1986.
Hungerford,
D.A. and Snyder, R.L.: Chromosomes of the rock hyrax, Procavia capensis
(Pallas), 1767. Experientia 25:870, 1969.
Kayanja,
F.I. and Sale, J.B.: The ovary of rock hyrax of the genus Procavia. J.
Reprod. Fertil. 33:223-230, 1973.
Kingdon,
J.: East African Mammals. Vol. I, pp. 329-339. Academic Press, London,
1971.
Kirkman,
S., Wallace, E.D., van Aarde, R.J. and Potgieter, H.C.: Steroidogenic
correlates of pregnancy in the rock hyrax (Procavia capensis).
Life Sci. 68:2061-2072, 2001.
Kleinschmidt,
T. and Braunitzer, G.: The primary structure of hemoglobins of the rock
hyrax Procavia habessinica, Hyracoidea): insertion of glutamine
in the alpha chains. Hoppe Seylers Z. Physiol. Chem. 364:1303-1313, 1983.
Kleinschmidt,
T., Czelusniak, J., Goodman, M. and Braunitzer, G.: Paenungulata: a comparison
of the hemoglobin sequences from elephant, hyrax, and manatee. Mol. Biol.
Evol. 3:427-435, 1986.
Makawiti,
D.W., Osaso, J. and Gombe, S.: In vitro metabolism of progesterone by
peripheral blood of rock hyrax (Procavia capensis). Gen. Comp.
Endocrinol. 83:159-163, 1991.
McNairn,
I.S. and Fairall, N.: Metabolic rate and body temperature of adult and
juvenile hyrax Procavia capensis. Comp. Biochem. Physiol. A 79:539-545,
1984.
Millar,
R.P. and Aehnelt, C.: Application of ovine luteinizing hormone (LH) radioimmunoassay
in the quantitation of LH in different mammalian species. Endocrinology
101:760-768, 1977.
Morsy,
T.A., al Dakhil, M.A. and el Bahrawy, A.F.: Characterization of Leishmania
aethiopica from rock hyrax, Procavia capensis trapped in Najran,
Saudi Arabia. J. Egypt. Soc. Parasitol. 27:349-353, 1997a.
Morsy,
T.A., al Dakhil, M.A. and el Bahrawy, A.F.: Natural leishmania infection
in rock hyrax, Procavia capensis (Pallas, 1766) order: Hyracoidea, trapped
in Najran, Saudi Arabia. J. Egypt. Soc. Parasitol. 27:75-81, 1997b.
Mossman,
H.W. and Duke, K.L.: Comparative Morphology of the Mammalian Ovary. University
of Wisconsin Press, Madison, 1973.
Müller,
R., Rüedi, D. and Hörning, B.: Magenparasiten bei Schliefern.
In, L.A. Page: Wildlife Diseases. Pp. 69-71. Plenum Press, NY, 1976.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Murray,
G.N.: The gestation period of Procavia capensis (dassie). J. S.
Afr. Vet. Med. Assoc. 13:27, 1942.
O'Donoghue,
P.N.: Reproduction in the female hyrax (Dendrohyrax arborea ruwenzorii).
Proc. Zool. Soc. London 141:207-237, 1963.
Oduor-Okelo,
D., Musewe, V.O. and Gombe, S.: Electron microscopic study of the chorioallantoic
placenta of the rock hyrax (Heterohyrax brucei). J. Reprod. Fertil.
68:311-316, 1983.
Ozawa,
T., Hayashi, S. and Mikkelson, V.M.: Phylogenetic position of mammoth
and Steller's sea cow within Tethytheria demonstrated by mitochondrial
DNA sequences. J. Mol. Evol. 44:406-413, 1997.
Prinsloo,
P. and Robinson, T.J.: Geographic mitochondrial DNA variation in the rock
hyrax, Procavia capensis. Mol. Biol. Evol. 9:447-456, 1992.
Quay, W.B.: A mid-aqueductal ependymal organ in the brain of the hyrax
(Procavia capensis). J. Comp. Neurol. 142:249-253, 1971.
Rehg,
J.E., Burek, J.D., Strandberg, J.D. and Montali, R.J.: Hemochromatosis
in the rock hyrax. In, R.J. Montali & G. Migaki, eds.: The Comparative
Pathology of Zoo Animals. Pp. 113-120, Smithsonian Institution Press,
Washington, DC, 1980.
Rubsamen,
K., Hume, I.D. and von Engelhardt, W.: Physiology of rock hyrax. Comp.
Biochem. Physiol. A 72:271-277, 1982.
Sale,
J.B.: Gestation period and neonatal weight of the hyrax. Nature 205:1240-1241,
1965.
Schoot,
van der, P.: Foetal genital development in Hyrax capensis, a species
with primary testicondia: proposal for evolution of Hunter's gubernaculums.
Anat. Rec. 244:386-401, 1996.
Soma,
H., Spraker, T. and Benirschke, K.: Macerated monozygotic twins in quadruplet
pregnancy of the Cape hyrax (Procavia capensis). J. Mammal. Soc.
Japan 6:210-213, 1976.
Stanhope,
M.J., Madsen, O., Waddell, V.G., Cleven, G.C., de Jong, W.W. and Springer,
M.S.: Highly congruent molecular support for a diverse superordinal clade
of endemic African mammals. Mol. Phylogenet. Evol. 9:501-508, 1998.
Thursby-Pelham,
D,.: The placentation of Hyrax capensis. Phil. Trans. Roy. Soc.
London, Series B. 213:1-20, 1924.
Wislocki,
G.B.: On an unusual placental form in the Hyracoidea: its bearing on the
theory of the phylogeny of the placenta. Contrib. Embryol. 21:83-95, 1930.
Wislocki,
G.B. and van der Westhuysen, O.P.: The placentation of Procavia capensis,
with a discussion of the placental affinities of the hyracoidea. Contrib.
Embryol. Carnegie Inst. 28:(171)67-88, 1940.
|