|
(Clicking
on the thumbnail images will launch a new window and a larger version
of the thumbnail.)
|
| Last updated: July 10, 2004. |
(Bolivian red Howler monkey)
Alouatta seniculus sara
Order: Primates
Family: Cebidae
1) General Zoological Data
Alouatta is the most widely distributed genus "of neotropical primates, with a geographic range extending from Mexico to northern Argentina" (Lima & Seuanez, 1991). It is also one of the largest cebids and is relentlessly pursued for food in South America. Thus, many species and subspecies are in peril of extinction. Just how many species there are, and which should be nominated as subspecies, are questions that are under frequent and continuing debate. An example is had in the discussion by Smith (1970) who described sympatry of two different, yet nominally different subspecies of black howler monkeys in Mexico. Howlers differ is size and, especially, in pelage. But coloration is not necessarily a good means of assigning species name, as was alluded to by Lima & Seuanez (1991). Moreover, there is extensive karyotypic evolution in this genus. Thus, precise point of origin is perhaps the best indicator for species designation. This is best brought out in the detailed discussion of the various species in Groves' new book (2001). He referred to "the xxx group" (e.g. "the seniculus group") and then assigns specific localities to the nominated species. Nowak (1999) listed nine species of howler monkeys, others consider there to be ten. Molecular studies by Harana et al. (1995) found evidence that links Ateles and Alouatta into one subfamily, the Atelinae. Hugot (1998) came to similar conclusions from studying the specific pinworms (Oxyuris) in the platyrrhine monkeys and considered these to have been co-evolved with platyrrhine speciation. Using mitochondrial and nuclear genes, Cortes-Ortiz et al. (2003) suggested that "the contemporary howler monkey species originated in the late Miocene and Pliocene, not the Pleistocene". mtDNA was more declarative than nuclear genes and they differentiated "cis- and trans-Andean clades". According to Gotch (1979), Alouatta is a combination of French and Latin to denote "a lot of shaggy hair".
The weight of male howler monkeys is usually larger than that of females but there is considerable variation. Generally speaking, weights between 4.4 and 11.5 kg have been listed for adults. The howler monkey is strongly prehensile with a very strong tail, is a preferably arboreal species, and it is the most folivorous of the South American primates. But fruit and other substances (nuts, blossoms) are also consumed. Fig trees are an important resource. In a detailed study of food consumed by red-handed howler monkeys, de Souza et al. (2002) described geophagy as also occurring in the wild. Howler monkeys, of course, are characterized by their loud howl - penetrating noises in the jungle. Having (inadvertently) rested under a tree with a band of howlers beginning their morning chatter, I can attest to the fear they may elicit. Jones (1980) lists 12 years as the longevity for a specimen of Alouatta villosa palliata.
 |
Alouatta seniculus sara at San Diego Zoo. |
Usually a single young is born after a gestation lasting around 180 days, and having a weight between 275 and 400 g. Shoemaker (1979) reviewed the captive reproduction in his zoo of a group of black howler monkeys and suggested from his literature review a gestational length of 187 days (139 in another paper) and 180-186 days for Alouatta palliata. Later (1982) he indicated that first successful keeping of howler monkeys in zoos did not occur until 1975, when the diet and other factors were better understood.
3) Implantation
No early implantation stages have been described.
4)
General Characterization of the Placenta
Mossman (1987) stated that only few placentas of South American primates
had been evaluated and he then described the placenta of Alouatta
as being "discoid", with other cebids having two disks (see
the chapters on Spider monkey and Saki monkey). He also alluded to a trabecular
structure of the villi. This is not so much the case for the howler monkey,
I believe. Its structure is much more villous than that of the callithrichids,
although the subchorial villi have a filiform appearance, similar to those
of the saki monkey. The villi are extremely cellular in this immature
placenta, the only one that was available to me, and there are no hematopoietic
islands in the villi. There are, however, nucleated red cells in the fetal
circulation, as behooves such an immature gestation. The floor of the
placenta has a thick layer of extravillous trophoblast that is adjacent
to the often degenerating decidua basalis without apparently invading
it, however. The single immature placenta I have had available for histology
had only a single disk. It weighed 30 g with an accompanying fetus weighing
90 g. The umbilical cord was 12 cm long.
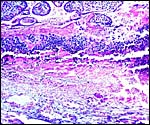
This placenta has a typical hemochorial relationship, with the villi covered by syncytium and directly bathed in maternal intervillous blood. Cytotrophoblast is already quite inapparent at this stage, but isolated cells may be found beneath the syncytium. Occasional syncytial "knots" are present. The fetal capillaries are close to the trophoblast; Hofbauer cells (macrophages) are present in the villous cores.
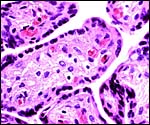 |
Slightly immature villi of Howler monkey with numerous fetal capillaries, occasional Hofbauer cells, and surface syncytiotrophoblast. |
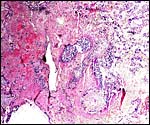
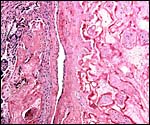
The umbilical cord of howler monkeys has not been studied, so far as I can tell. Young (1972) when seeking to identify the different types of anastomoses between the umbilical arteries in the cord ("Hyrtl's anastomosis") did not have howler monkeys available and found different anastomotic features in various other cebids. Many of the latter, however, also had four vessels in their cord, 2 arteries and 2 veins, as is shown for the single Alouatta seniculus sara cord available to me. It is similar in that respect to the umbilical cord of spider monkeys (see chapter on Ateles), except that there is no patent allantoic duct. Only tiny discontinuous remnants of the duct are found in the center between the umbilical arteries, as is also common in humans. Spatz (1968) related the length of primate umbilical cords to the overall length of neonates in numerous species and had seven howler monkey umbilical cords available. He determined the relationship to be similar to that of rhesus monkeys. There is no squamous metaplasia on the umbilical cord surface of this 12 cm long cord from the abortus studied here. It had a counterclockwise (right) twist.
 |
Two cross sections of Howler monkey umbilical cord (Alouatta seniculus sara). A tiny remnant of allantoic duct can be seen in the cord section on the left; it is between the thicker arteries. |
No published studies are known to me.
8)
Extraplacental membranes
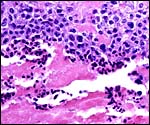
I have only one section available of the membranes from the lateral aspect
of the immature placenta described above. It is shown next and, in contrast
to some other primates, villi extend to the edge, and they are found to
atrophy there beneath the chorion. No allantoic sac is present and the
amnion is lined by a very thin epithelium without metaplastic change.
It is avascular.
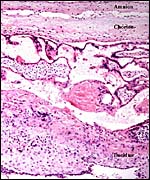 |
Margin of immature placenta with origin of free membranes. There is a decidua capsularis, quite similar to human gestations and atrophying villi are found. |
There is no apparent infiltration of trophoblast deep into the endometrium, let alone into the myometrium. Only a thin layer of superficial decidua is infiltrated by extravillous trophoblast. There is no knowledge as to possible maternal vascular infiltration by trophoblast.
10)
Endometrium
There is little endometrial decidual transformation at the base, where
much necrosis occurs and where considerable superficial fibrinoid is found.
Decidua capsularis forms as is shown above.
11)
Various features
No subplacenta exists.
12)
Endocrinology
Herrick et al. (2000) studied the estrous cycle by RIA of urinary unconjugated
progesterone. The approximate length of the cycle was found to be 29 days.
Glander (1980) had earlier reported a 16 day cycle in free-ranging animals.
Fecal testosterone was measured by Moreland et al. (2001) who also gave
specifics on methods and results of electroejaculation. Their study was
undertaken in order to assist with the potential aid to an endangered
group of animals. I am unable to find publications on gonadotropins or
measurements of estrogens.
13) Genetics
The karyotypes of howler monkeys are complex, with chromosome numbers
ranging from 42 to 53, different species having different numbers. It
is possible that hybrids among species or subspecies occur, but they were
not considered in the book by Gray (1972), nor are they mentioned in other
publications. This group of species is also characterized by the occurrence
of Y-autosome fusions, microchromosomes, and multiple sex chromosome systems
occurring in a variety of species. Here follows a possibly incomplete
list of chromosome numbers from various publications in chronological
order:
| Alouatta
caraya Alouatta seniculus Alouatta villosa (?=palliata) Alouatta fusca clamitans Alouatta caraya Alouatta palliata Alouatta seniculus seniculus Alouatta caraya Alouatta seniculus sara Alouatta seniculus stramineus Alouatta seniculus macconnelli Alouatta seniculus sara Alouatta seniculus arctoidea Alouatta caraya Alouatta caraya Alouatta palliata Alouatta seniculus sara Alouatta seniculus straminea |
2n=52 (Egozcue & Egozcue, 1966) 2n=44 (Bender & Chu, 1963) 2n=53 (Hsu, 1965) 2n=50 (& 49) (Koiffman & Saldanha, 1974) 2n=52 (Koiffman & Saldanha, 1974) 2n=53 (Jones et al., 1975, Ma et al., 1975) 2n=44 (43-45) (Yunis et al., 1976) 2n=52 (Mudry et al., 1981) 2n=48-51 (Minezawa et al., 1985) 2n=47, 48, 49 (Lima & Seuanez, 1991) 2n=47, 48, 49 (cited by Lima & Seuanez, 1991) 2n=42 (Consigliere et al., 1996) 2n=43 (Consigliere et al., 1996) Multiple sex chromosomes (Rahn et al., 1996) 2n=52 (Mudry et al., 1998) 2n=53 (At CRES) 2n=49-52 (At CRES) 2n=48 (46) (At CRES) |
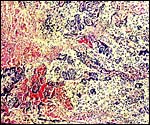
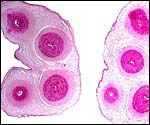
James et al. (1997) examined 36 allozyme loci and RFLP loci of mtDNA in a group of A. pigra and found surprisingly little genetic heterogeneity in this group. Bonvincino et al. (2001) also studied genes (cytochrome bDNA) in several species of howler monkeys. They concluded that the unusual sex chromosome constitution described earlier appeared independently three times during the radiation of this species. Importantly, Mudry et al. (2001) studied the meiotic paring of chromosomes in two howler monkey species, and demonstrated the breakpoint by chromosome painting.
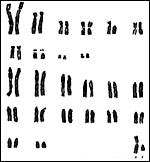 |
Karyotype of male Alouatta seniculus sara, 2n=50 from San Diego Zoo. |
Multiple autoantibodies (rheumatoid factor, antinuclear antibody, antithyroglobulin, heterophil agglutinins and false VDRL) were identified in eight howler monkeys studied by Persellin & Chang (1974). I know of no other specific studies, except for the serologic surveys listed below.
15)
Pathological features
As is true of many catarrhine and platyrrhine monkeys, Howler monkeys
often have focal infarcts in their placenta. These are very similar to
those found in human gestations where they are commonly due to pre-eclampsia
with altered decidual blood vessels. No such blood vessel changes have
been described in these monkeys, however.
In 1998 a yellow fever virus epidemic killed numerous howler monkeys in
eastern Amazonia (Mondet et al., 2002). The frequency of toxoplasmosis
in howler monkeys was assessed by a serologic survey of numerous animals
in French Guiana (Carme et al., 2002). Howlers, as well as other tree-dwelling
species, had a low prevalence of antibodies to toxoplasma. At the Audubon
Park, New Orleans, a howler monkey died from eosinophilic meningitis due
to Angiostrongylus cantonensis infection (Gardiner et al., 1990).
Pope (1966) identified lice, pinworms and a tapeworm in black howler monkeys.
Salmonella typhimurium was isolated from an abscess in a black
howler monkey by Kourany & Rossan (1971). Maruffo et al. (1966) found
lipofuscin liver pigmentation in nearly all of the 290 free-ranging howler
monkeys, with occasional fatty infiltration and rarely attended by necrosis.
Experimental infection with Herpes saimiri virus caused death in
howler monkeys (Rangan et al., 1977). Maruffo & Malinow (1966) reported
on a seminoma in one of 314 black howler monkeys studied, and later, Maruffo
(1967) identified adrenal adenomas and a renal adenoma in a howler monkey.
Scott (1992) described a dissecting aortic aneurysm. Others have also
published on cardiac and vascular disease in howlers. Thus, Maruffo &
Malinow (1967) examined 280 free-ranging black howler monkeys and found
pericarditis in 20, myocarditis in 19, and endocarditis in 2 animals.
Malinow & Maruffo (1965) identified aortic sudanophilia, streaks and
small atheromas in the same group of animals autopsied.
Anesthesia and its reversal have been described in detail by Vie et al. (1998b). The same group of investigators recorded the hematologic findings and serum chemistry of red howlers (Vie et al., 1998a). Clark et al. (1987) examined plasma lipoproteins and found them similar to humans, while fatty acids were very low. The blood groups of howler monkeys were studied by Froehlich et al. (1977), and the prevalence of blood group "B" of South American monkeys was upheld. Their serum contained anti-A, and the saliva had H and B antigens.
17)
Other resources
Various fibrous tissue cell lines are available from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder (oryder@ucsd.edu).
The names of species available are at the end of the list above.
18)
Other remarks - What additional Information is needed?
Implantation needs to be studied and some mature placentas need to be
described, specifically to rule out bilobation of the placental disks.
The length of the umbilical cord is not firmly established. We also need
information of trophoblast infiltration into decidua and maternal vessels.
Acknowledgement
The animal photograph in this chapter comes from the Zoological Society
of San Diego
References
Bender, M.A. and Chu, E.H.Y.: The chromosomes of primates, in, J. Buettner-Janusch,
ed.: Evolutionary and Genetic Biology of Primates. Academic Press, N.Y.
Bonvincino, C.R., Lemos, B. and Seuanez, H.N.: Molecular phylogenetics of howler monkeys (Alouatta, Platyrrhini). A comparison with karyotypic data. Chromosoma 110:241-246, 2001.
Carme, B., Aznar, C., Motard, A., Demar, M. and de Thoisy, B.: Serologic survey of Toxoplasma gondii in noncarnivorous free-ranging neotropical mammals in French Guiana. Vector Borne Zoonotic Dis. 2:11-17, 2002.
Clark, S.B., Tercyak, A.M. and Glander, K.E.: Plasma lipoproteins of free-ranging howling monkeys (Alouatta palliata). Comp. Biochem. Physiol. B 88:729-735, 1987.
Consigliere, S., Stanyon, R., Koehler, U., Agoramoorthy, G. and Wienberg, J.: Chromosome painting defines genomic rearrangements between red howler monkey subspecies. Chromosome Res. 4:264-270, 1996.
Consigliere, S., Stanyon, R., Koehler, U., Arnold, N. and Wienberg, J.: In situ hybridization (FISH) maps chromosomal homologies between Alouatta belzebul (Platyrrhini, Cebidae) and other primates and reveals extensive interchromosomal rearrangements between howler monkey genomes. Amer. J. Primatol. 46:119-133, 1998.
Cortes-Ortiz, L., Bermingham, E., Rico, C., Rodriguez-Luna, E., Sampaio, I. And Ruiz-Garcia, M.: Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Mol. Phylogenet. Evol. 26:64-81, 2003.
Egozcue, J. and de Egozcue M.W.: The chromosome complement of the howler monkey (Alouatta caraya, Humboldt 1812). Cytogenetics 5:20-27, 1966.
Froehlich, J.W., Socha, W.W., Wiener, A.S., Moor-Jankowski, J. and Thorington, R.W.: Blood groups of the mantled howler monkey (Alouatta palliata). J. Med. Primatol. 6:219-231, 1977.
Gardiner, C.H., Wells, S., Gutter, A.E., Fitzgerald, L., Anderson, D.C., Harris, R.K. and Nichols, D.K.: Eosinophilic meningoencephalitis due to Angiostrongylus cantonensis as the cause of death in captive non-human primates. Amer. J. Trop. Med. Hyg. 42:70-74, 1990.
Glander, K.E.: Reproduction and population growth in free-ranging mantled howling monkeys. Amer. J. Phys. Anthropol. 53:25-36, 1980.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Groves, C.: Primate Taxonomy. Smithsonian Institution, Washington, D.C. 2001.
Harada, M.L., Schneider, H., Schneider, M.P., Sampaio, I., Czelusniak, J. and Goodman, M.: DNA evidence on the phylogenetic systematics of New World monkeys: support for the sister-grouping of Cebus and Saimiri from two unlinked nuclear genes. Mol. Phylogenet. Evol. 4:331-349, 1995.
Herrick, J.R., Agoramoorthy, G., Rudran, R. and Harder, J.D.: Urinary progesterone in free-ranging red howler monkeys (Alouatta seniculus): preliminary observations of the estrous cycle and gestation. Amer. J. Primatol. 51:257-263, 2000.
Hsu, T.C.: Cited by: de Boer, L.E.M.: Cytotaxonomic Studies in the Primate Suborders Prosimii and Platyrrhini.Drukkerij Bronder-Offset B.V., Rotterdam, 1974.
Hugot, J.P.: Phylogeny of neotropical monkeys: the interplay of morphological, molecular, and parasitological data. Mol. Phylogenet. Evol. 9:408-413, 1998.
James,
R.A., Leberg, P.L., Quattro, J.M. and Vrijenhock, R.C.: Genetic diversity
in black howler monkeys (Alouatta pigra). Amer. J. Phys. Anthropol.
102:329-336, 1997.
Jones, M.L.: Lifespan in mammals. In, The Comparative Pathology of Zoo
Animals. R.J. Montali and G. Migaki, eds. Pp. 495-515.Smithsonian Institution
Press, Washington D.C. 1980.
Jones, T.C., Thorington, R.W., Miller, A. and Morgan, L.: Y-autosome translocation in the howler monkey, Alouatta palliata. J. Med. Primatol. 4:299-307, 1975.
Koiffman, C.P. and Saldanha, P.H.: Cytogenetics of Brazilian monkeys. J. Hum. Evol.3:275-282, 1974
Kourany,
M. and Rossan, R.N.: A subcutaneous abscess associated with Salmonella
typhimurium in a black howler monkey (Alouatta villosa). Lab.
Anim. Sci. 21:412-414, 1971.
Lima, M.M. and Seuanez, H.N.: Chromosome studies in the red howler monkey,
Alouatta seniculus stramineus (Platyrrhini, Primates): description
of an X1X2Y1Y2/X1X1X2X2 sex-chromosome system and karyological comparisons
with other subspecies. Cytogenet. Cell Genet. 57:151-156, 1991.
Ma, N.S.F., Jones, T.C., Thorington, R.W., Miller, A. and Morgan, L.: Y-autosome translocation in the howler monkey, Alouatta palliata. J. med. Primatol. 4:299-307, 1975.
Malinow, M.R. and Maruffo, C.A.: Aortic atherosclerosis in free-ranging howler monkeys (Alouatta caraya). Nature 205:948-949, 1965. (see also: Naturally occurring atherosclerosis in Howler monkeys (Alouatta caraya. J. Atheroscleros. Res. 8:368-380, 1966).
Maruffo, C.A.: Spontaneous tumours in howler monkeys. Nature 213:521, 1967.
Maruffo, C.A. and Malinow, M.R.: Seminoma in a howler monkey (Alouatta caraya). J. Pathol. Bacteriol. 91:280-282, 1966.
Maruffo, C.A. and Malinow, M.R.: Pathologic changes of the heart in free-ranging howler monkeys (Alouatta caraya, Humboldt, 1812). Amer. J. Vet. Res. 28:237-243, 1967.
Maruffo, C.A., Malinow, M.R., Depaoli, J.R. and Katz, S.: Pigmentary liver disease in howler monkeys. Amer. J. Path. 49:445-456, 1966.
Minezawa, M., Harada, M., Jordan, O.C. and Borda, C.J.V.: Cytogenetics of Bolivian endemic red howler monkeys (Alouatta seniculus sara): accessory chromosomes and Y-autosome translocation related numerical variations. Kyoto University Overseas Research Report on New World Monkeys. 5:7-16, 1985.
Mondet, B., Vasconcelos, P.F., Travassos da Rosa, A.P., Travassos da Rosa, E.S., Rodrigues, S.G., Travassos Rosa, J.F. and Bicout, D.J.: Isolation of yellow fever virus from nulliparous Haemagogus (Haemagogus) janthinomys in eastern Amazonia. Vector Borne Zoonotic Dis. 2:47-50, 2002.
Moreland, R.B., Richardson, M.E., Lamberski, N. and Long, J.A.: Characterizing the reproductive physiology of the male southern black howler monkey, Alouatta caraya. J. Androl. 22:395-403, 2001.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Mudry de Pargament, M.D., Brieux de Salum, S. and Collilas, G.J.: Estudio cyogenetico del mono aullador Alouatta caraya de la Republica Argentina. Physis (Buenos Aires), Secc. C. 40 (98):63-70, 1981.
Mudry,
M.D., Rahn, M., Gorostiaga, M., Hick, A., Merani, M.S. and Solari, A.J.:
Revised karyotypes of Alouatta caraya (Primates: Platyrrhini) based
on synaptonemal complex and banding analyses. Hereditas 128:9-16, 1998.
Mudry, M.D., Rahn, M., and Solari, A.J.: Meiosis and chromosome painting of sex chromosome systems in Ceboidea. Amer. J. Primatol. 54:65-78. 2001.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
De Oliveira, E.H., Neusser, M., Figueiredo, W.B., Nagamachi, C., Pieczarka, J.C., Sbalqueiro, I.J., Wienberg, J. and Mueller, S.: The phylogeny of howler monkeys (Alouatta, Platyrrhini): reconstruction by multicolor cross-species chromosome painting. Chromosome Res. 10:669-683, 2002.
Persellin, R.H. and Chang, R.J.: Multiple IgM autoantibodies in a nonhuman primate. Inter. Arch. Allergy 46:815-821, 1974.
Pope, B.L.: Some parasites of the howler monkey of northern Argentina. J. Parasitol. 52:166-169, 1966.
Rahn, M.I., Mudry, M., Merani, M.S. and Solari, A.J.: Meiotic behavior of the X1X2Y1Y2 quadrivalent of the primate Alouatta caraya. Chromosome res. 4:350-356, 1996.
Rangan, S.R., Martin, L.N., Enright, F.M. and Abee, C.R.: Herpesvirus saimiri-induced lymphoproliferative disease in howler monkeys. J. Natl. Cancer Inst. 59:165-171, 1977.
Scott, G.B.D.: Comparative Primate Pathology. Oxford University Press, 1992.
Shoemaker, A.H.: Reproduction and development of the black howler monkey Alouatta caraya at Columbia zoo. Internat. Zoo Yearbk. 19:150-155, 1979.
Shoemaker, A.H.: Fecundity in the captive howler monkey, Alouatta caraya. Zoo Biol. 1:149-156, 1982.
Smith, J.D.: The systematic status of the black howler monkey, Alouatta pigra Lawrence. J. Mammal. 51:358-369, 1970.
De Souza, L.L., Ferrari, S.F., da Costa, M.L. and Kern, D.C.: Geophagy as a correlate of folivory in red-handed howler monkeys (Alouatta belzebul) from eastern Brazilian Amazonia. J. Chem. Ecol. 28:1613-1621, 2002.
Spatz, W.B.: Nabelschnur-Längen bei Insektivoren und Primaten. Z. Säugetierk. 33:226-239, 1968.
Vie, J.C., Moreau, B., de Thoisy, B., Fournier, P. and Genty, C.: Hematology and serum biochemistry values of free-ranging red howler monkeys (Alouatta seniculus) from French Guiana. J. Zoo Wildl. Med. 29:142-149, 1998a.
Vie, J.C., De Thoisy, B., Fournier, P, Fournier-Chambrillon, C., Genty, C. and Keravec, J.: Anesthesia of wild red howler monkeys (Alouatta seniculus) with medetomidine/ketamine and reversal by atipamezole. Amer. J. Primatol. 45:399-410, 1998b.
Young, A.: The primate umbilical cord with special reference to the transverse communicating artery. J. Human Evol. 1:345-359, 1972.
Yunis, E.J., Torres de Caballero, O.M., Ramirez, C. and Ramirez Z.E.: Chromosomal variations in the primate Alouatta seniculus seniculus. Folia Primatol. 25:215-224, 1976.