| March 217 |
Equus caballus by
Manu Sebastian, Lexington, KY
&
Kurt Benirschke, San Diego, CA
Order:
Perissodactyla
Family: Equidae
1) General Zoological Data
The ancestry of horses from five-toed species via the small Eohippus of the American continent has been a prime topic of study for evolutionary
biologists. It is best traced in two contributions: by Simpson (1951),
and by Thenius & Hofer (1960). Coming from the American continent,
the equid ancestors distributed to Eurasia while speciating into the extant
members during their expansion to the West and into Africa. In the process,
chromosomal fusions occurred in the equidae, causing karyotypes to evolve
that range from 66 to 32 chromosomes, whilst preserving the same amount
of DNA. The current domestic horse is thought to have derived from the
Mongolian wild horse, Equus przewalskii. Other authors, however,
have considered the extinct Tarpan to be the ancestral species of the
domestic horses. A very large number of different horse breeds have been
produced during their domestication, ranging from large tract animals
to very small ponies.
Somewhat different views of the speciation of equidae are expressed in various chapters of the new book by Bowling & Ruvinsky (2000) that need to be consulted for details.
 |
Norwegian Fjord Pony with shorn mane. |
 |
Przewalski's horse stallion with "standing mane" and "butterfly" belly, perhaps the ancestor of the modern domestic horse. |
2)
General Gestational Data
There is an abundance of gestational data on horse reproduction. They are
summarized in many books and chapters, e.g. that by Samper (2000). Additionally,
some information on equine placentas is contained in my chapters on Grevy's
zebra and the Kiang. One of the most comprehensive books on the horse biology,
however, is that edited by McKinnon & Voss (1992). It contains every
aspect of equine reproduction and its ramifications. For specific details,
I recommend the reader consult this large volume.
Gestation in horses is seasonal and lasts around 330 to 340 days. The length
apparently depends largely on the specific breed. Because there is such
enormous difference in the sizes and weights of these animals from different
breeds, no data are given here of these features. The interested reader
may find the extremes in the data supplied from the most interesting embryo
transfer experiments by Allen et al. (2002a,b). One author (Caslick, 1932)
found an average placental weight of 5,700g, while Prickett (1970) gives
weights from 4,500 to 8,200 g. More detailed information on reproductive phenomena may be found also in chapters contained in the book by Bowles & Ruvinsky (2000).
3) Implantation
Implantation and development of the placenta in the horse has probably been
more extensively studied than that of most other domestic animal species.
A really comprehensive description came originally from Amoroso (1961),
with many other authors filling in finer details. Steven (1982) has written
another most easily comprehended description of the horse placenta; it also
reviews the long history preceding his detailed writings. Moreover, this
description is accompanied by excellent drawings. The most recent comprehensive
discussion on equine placentation is found by Wooding & Flint (1994).
The same volume also contains abundant endocrine and fine structural information.
Mossman (1987) has a surprisingly superficial description of equid membranes
and it differs frequently from that found by other students. Furthermore,
his drawing does not conform to what is now known of the equine placentation.
Specifically, the site of cord insertion is inaccurate. Ramsey (1982), on
the other hand, has a very concise chapter on horse placentation that is
worth reading for initial orientation. The most recent comprehensive details
of horse placentation come from writings by Allen & Stewart (2001) whose
laboratory has perhaps contributed the most information to our understanding
of this interesting organ.
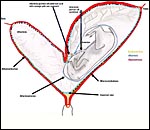
The equine gestation is unusual in many ways. First of all, it has this
unusually long preimplantation development of the massively enlarging blastocyst;
it is suspended for days in a large amount of "uterine milk" (glandular
secretion) that supports the trophoblast. This early spherical embryo is
also unusual in that it possesses a hard secondary membrane beneath the
zona pellucida (Betteridge et al., 1982) that lasts to about day 25. The
conceptus is being shuttled back and forth in the uterine horns for at least
a week before it finally becomes "fixed", usually in one horn,
on day 15 to 17 (see Ginther, 1985). Here, the embryo comes to lie at an
antimesometrial site and is contained in a recess that Enders & Liu
(1991a) referred to as the "implantation chamber". Occasional
"body implantation", in the middle of the uterus, occurs. The
placenta ultimately extends into both uterine horns and is studded on its
external surface by villi in the form of "microcotyledons". This
term has become used customarily; it may be confusing to the novice, as
the placenta looks externally, at least superficially, as though it is covered
by a myriad of small villi. The villous projections interdigitate with the
maternal endometrial folds and glands in a very complex manner. This, again,
is diagrammed beautifully by Steven (1982). In contrast to the more uniform
villous projections found in pig placentas, villous stems are produced in
the horse ("microcotyledons" - 1 to 2 mm in size) that branch
two or three times and which are separated from one another by intervillous
stretches of "areolae or arcades or areolae". Vascularization
of the endometrium in these areas also differs. The first microcotyledons
can be recognized by day 61. The relation to maternal epithelium is epithelio-chorial
without invasion in all these places. It is well shown in my chapter on
Grevy zebra where an implanted placenta was available for study. It is generally
similar to that of horses. Most gaseous and water exchange between mare
and fetus occurs through the microcotyledonary trophoblast. Iron, and several
proteins and some other substances are transferred by endometrial secretions
in the areolae. Therefore, these regions also have a significantly different
histologic appearance. This has been beautifully studied with histochemistry
and fine structural details by Wooding et al. (2000).
The only region of invasion that occurs in equine placentas takes place
at the "chorionic girdle" with the formation of "endometrial
cups", now shown to be the result of trophoblast modification.
Their history and development have been extensively recapped by Allen (1982).
The girdle is located at the interface between the expanding allantochorion
and the regressing yolk sac, around the future site of insertion of the
umbilical cord and towards the lower aspect of the pregnant horn. At that
time, the placenta fills only one of the uterine horns, later it expands
into both. These regions are the site of eCG (formerly PMSG) hormone production
and they are temporary features. Enders & Liu (1991b) studied this process
electronmicroscopically and later referred to the region as producing endocrine
and exocrine secretions (a yellow viscid fluid) (Enders et al., 2000). At
term, however, one is still able to see the remnants of this girdle around
the insertion site of the cord as a thin band. The girdle is mature on day
35 and it's then binucleate, epithelioid trophoblastic cells begin to invade
though the endometrial epithelium into the endometrial stroma. Here they
engender a marked lymphocytic-plasmacellular and histiocytic reaction. This
inflammatory reaction, however, is primarily expansive towards the end of
the production phase of eCG. That the hormone production is located here
was shown by extraction, by cytochemical reactions and was also proven from
extraction of tissue-cultured cup cells. eCG persists for variable lengths.
By day 120-130 the cups have involuted and then undergo necrosis. Thereafter,
the necrotic debris may be present in small sacs or pouches that often project
into the allantochorion. The pouches contain necrotic debris and must not
be confused with hippomanes (Mossman, 1987). Much speculation is had as
to the nature of the regression; is it "rejection" or are processes
of apoptosis in play? Other processes have been invoked as well (lipid degeneration)
to explain the means by which the reduced vascular supply leads to the degeneration
(Enders et al., 2000). The fine structural study by Wooding et al. (2001)
localized the eCG production to the heavily endowed cytoplasm of the cup
cells and suggested that no hormonal stimulus causes the "constitutive" secretion of these unique cells.
Much interest has focused on the cup formation of different equines (smaller
in donkeys) and the corresponding amount of eCG production. Not only has
this been studied in various equidae, it has been examined especially well
in embryo transfers and hybrids (see Allen, 1982; Allen et al., chapter
63 in McKinnon & Voss, 1993). As the girdle is large in horses than
in donkeys, it was interpreted to be under genetic control of the paternal
genotype. More recent studies of appropriate embryo transfer experiments
carried out by Allen et al. (2002c) have been reported so far in abstract
form only. They indicated an overriding influence of the maternal endometrial
genotype in determining the size and development of the cups. Their final
publication is awaited with great interest. Other factors now need also
consideration, as the epigenetic aspects of imprinting that make reciprocal
hybrids develop such markedly different phenotypes (Pennisi, 2001). The
microscopic changes occurring at the girdle were well-studied in the fine
structural analysis conducted by Enders et al. (1990). Much investigation
has also focused on the relationship of this invasive trophoblast and the
apparently immunologic reaction to the paternal MHC antigen composition,
especially in the donkey-in-horse model (see Allen, 1982; Baker et al.,
2001). The topic is still too unresolved and much too complex for a detailed
consideration in this short review. It needs to be read in the original
publications.
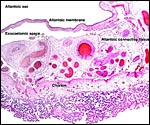
Although late in development, the allantoic sac becomes very large and contains
fetal urine and hippomanes as characteristic components. A yolk sac develops
early, is large at first but then involutes at the end of the first month
of gestation. Its remnants can always be found in a much atrophied state
within the insertion of the umbilical cord at term.
At the College of Agriculture in Lexington , Kentucky , a comprehensive workshop was held in 2003 on “The Equine Placenta” (Powell, 2004). The superb book that derived from this workshop is richly illustrated and can be recommended for many further details.
4)
General Characterization of the Placenta
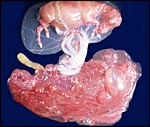
After all the mobility of the early conceptus and the very slow elongation
of the large round conceptus, at last, by day 100, the allantochorion
has filled both uterine horns. The outside is now studded with microcotyledons
and villi that originate from the allantochorionic membrane. Next in the
sequence of layers come the large allantoic blood vessels within the exocoelomic
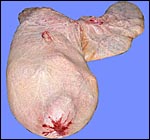
space and then, on the inside, is the allantoic cavity. The delivered
placenta is usually quite dark red when it is inverted so it would assume
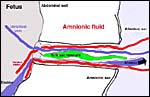
its position in utero. The amnionic cavity more or less floats freely
in the large allantoic sac that is filled with fetal urine. The allantoamnion
(allantois outside, amnion inside) is also unusually vascularized when
compared with other species. One has to assume that some fluid shifts
may take place between the two cavities and perhaps that this is enabled
by this extensive vasculature.
| Weights
and measurements of various equine placentas available |
|||
| Species | Weight | Umbilical cord | Fetus |
| Domestic horses | 5,700 g | 30.5 to 137.2 cm | (Caslick, 1932) |
| Thoroughbreds | 4,500 - 8,200 g | 36 to 84 cm | (Whitwell, 1975) |
| Quarterhorse | Fetal demise | 90 (30+60 cm) | 23,000 g (demise) |
| Appaloosa | 4,300 g | 40 cm (24+16 cm) | Healthy foal |
| Morgan | 2,200 g | 115 cm (53+62 cm) | Healthy foal-trauma) |
| Przewalski's horse | 1,900 g | 30 cm | |
| 2,600 g | |||
| 49 cm (21+28) | 4,840 g (in utero) | ||
| Somali wild ass | 2,180 g | 32 cm | |
| 38 cm | 15,700 g (in utero) | ||
| 1,925 g | 49 cm | Healthy foal | |
| Onager | 35 cm | ||
| 1,000 g | 11,475 g (stb.) | ||
| 2,000 g (twin) | 51 cm | ||
| 1,800 g | 55 cm | ||
| Kulan | 1,325 g | 80 cm | |
| 1,900 g | 38 cm | ||
| Kiang | 3,700 g | 67 cm | |
| 2,025 g | 41 cm | Weak foal | |
| 3,000 g | 84 cm | "Nipple" on amnion | |
| |
2,600 g | 45 cm | (2,250 g actual placental weight, rest is membranes) |
| Grevy's zebra | 1,580 g | 68 cm | |
| Grevy's zebra | 1,560 g | 68 cm | |
| Burchell's zebra | 1,200 g | 62 cm | |
| Damara zebra | 2,980 g | 58 cm | |
| Hartmann's zebra | 1,400 g | 68 cm | |
| Hartmann's zebra | 1,700 g | 55
cm |
|
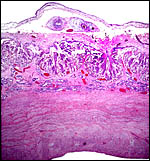
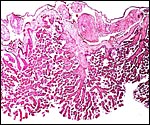
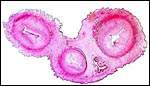
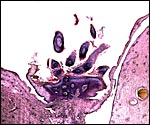
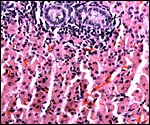
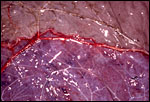
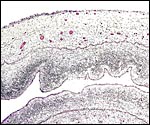
The surface of microcotyledons and their villous branches is a single layer of uninuclear trophoblast. Binucleate cells, as seen in ungulate placentas, are absent there; they are seen only at the girdle. There is no invasion of maternal tissue, rather, the trophoblast directly abuts the endometrial epithelium in a complex manner in the villous portions of the placenta. As pregnancy proceeds, the proximity of this exchange area increases and there is then a very close approximation of fetal and maternal capillaries. Only beneath the chorionic surface is the trophoblast more cylindrical and often vacuolated. Here also, some yellow pigmentary inclusions are found. They are iron-stain negative and may, as in ungulates, represent melanin, but other pigments cannot be excluded. We have not found this pigment further discussed in the equine literature except to note that, generally, it is assumed that iron is transported to the fetus with uteroferrin from endometrial secretions (Wooding et al., 2000). This cylindrical structure of the trophoblast is also apparent in between the microcotyledons, the areolae or arcades. It is assumed to have a histotrophic function for absorption of uterine secretions.
There is considerable variation in the finer structure and size of microcotyledons that has been assessed quantitatively by Wilsher & Allen (2003). Their extensive data show convincingly that there is a strong correlation of villous surface and fetal weight; primiparous mares (especially older primipara) had smaller foals, in part or perhaps wholly because of this smaller villous surface area. Various other quantitative data are given in this excellent contribution.
Jones et al. (2000) examined the pattern of glycosylation of the "interhemal barriers" of horse, donkey, and camel. This was similar between horse and donkey but much different in the camel. From this it was suggested that this may be important to prevent interspecific hybrids. Macdonald et al. (2000) studied the villous structures of the horse by scanning electron microscopy and published impressive, conclusive pictures of maternal and fetal aspects of the pony placentas. This suggested to them that the "microcotyledons" are the aggregates of encapsulation of 16 uterine crypt regions. With advancing gestation the villi kept branching until very close to term.
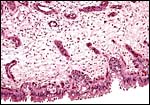
 |
In the more columnar, vacuolated trophoblast beneath the chorion of microcotyledons, often contents of yellow inclusions are found (at arrows). |
The umbilical cord of horses has been described in considerable detail by Whitwell (1975). It has a somewhat longer portion within the amnionic cavity and a shorter segment in the allantoic sac; but that varies. The membrane insertion delineates these two segments. There is a considerable variability in the length of the cord, with measurements from 36 to 84 cm in the thoroughbred foals (mean 55 cm) of her study. Excessive lengths have been considered to be the cause of abortion, of fetal strangulation, and of fetal demise (Whitwell, 1975; Caslick, 1932, others). The ultimate cause for this variability in cord lengths is unknown. Caslick measured lengths from 30.5 to 137.2 cm, with the variability perhaps being more distributed over the amnionic portion.
The length of the umbilical cord is surely determined in early gestation, as abortions with excessive lengths occur not uncommonly. Also, in contrast to many other species, the horse fetus has great mobility in early gestation. Similar variations in length occur in human placentation; and short cords are usually due to lack of fetal motion (Benirschke, 1994; Snider, 1997). One wonders whether mobility is perhaps in part the result of the "breed" of horse and notes that the variability in length of the horse cord is often in the amnionic portion. One can imagine that race horses might be more mobile, perhaps already in utero, than draught horses. Measurements comparing different "breeds" are outstanding but would be of great interest.
There is often considerable twisting of the horse umbilical cord. It has occasionally led to excessive torsion and strangulation, with impressions left on the stillborn foal (Whitwell, 1975; Hong et al.; Giles et al., 1993). Perhaps it is also the cause of some of the allantoic duct dilatations (6%) observed by Whitwell & Jeffcott (1975).
The latter authors found the cord to be inserted at the posterior aspect of the uterus, either in or near midline, or in the pregnant horn. The umbilical cord contains two arteries and one vein, in addition to a widely patent, thin-walled allantoic duct. It is accompanied by strands of smooth muscle fibers. While two major venous branches expand over the allantochorion, they fuse to become a single vein at the passing through the amnionic insertion around the cord. The allantoic portion of the umbilical cord is not a solid cord but a "web of vessels" (Whitwell, 1975). In addition to the three large vessels, numerous smaller blood vessels are also present in the umbilical cord, especially in the vicinity of the allantoic duct. Remnants of the vitelline duct may be present and Whitwell has identified remnants of the yolk sac in all placentas she has studied (personal communication). The cord may be significantly spiraled and, in the amnionic portion, it has many very small foci of sqamous metaplasia on its surface.
Absence of one umbilical artery was seen once in 145 cords with a fetus having renal anomalies. Furthermore, in excessively long umbilical cords and heavily spiraled ones, thrombosis and vascular calcification have been observed. The last kiang placenta observed had four umbilical vessels and no twists at all.
Steven (1982) showed an excellent diagram of the vasculature in the uterus that supplies the microcotyledons and the arcades. It has been repeated in many subsequent publications on the horse placenta. He depicted the straight maternal arteries to proceed between the microcotyledons whose branches then enter the endometrium that correspond to the microcotyledons. The veins draining this tissue pursue a central flow from the centers of the microcotyledons. Moreover, towards term, there is more and more "invagination or infiltration" of the fetal capillaries into the trophoblast so that, at the end of gestation, fetal and maternal capillaries come to lie in very close physical proximity. This assures maximal possibility of nutrient/gaseous exchange.
8)
Extraplacental membranes
A particularly detailed report on the membranes came from the study by
Whitwell & Jeffcott (1975). They found that the majority of placentas
are inverted at birth (villi inside), unless (in 24%) the nonpregnant
horn was of the same size as that containing the fetus. In that case,
the villi were usually external at delivery. These authors always identified
a small tubular yolk sac remnant near the cord insertion and found hippomanes
in the allantoic sac of all animals. In their experience, five regions
of the placental surface lack villous endowment. These are: the regions
of tubal origin, the "cervical star", and the area of the former
chorionic girdle, where no villi grow.
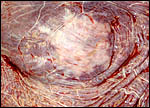
The cervical star requires some further discussion. It is visible as a
region without the red villi and has radiating, bare, white areas from
its center. Whitwell (personal communication, 2002) made scanning EM pictures
of the region. These showed a complete absence of villi and bare, thick
folds of connective tissue. If ascending infection were to take place
(the horse has a relatively short cervix and ascending infection is reasonably
common), the organisms would have to traverse the region of the cervical
star and would then come into very close contact with the allantoamnion.
As the amnion expands and the fetus grows with later gestation (than that
shown in the diagram above) the allantoamnion is closely pressed to the
region of the cervical star, the region that ruptures during spontaneous
delivery. Thus, infection of the amnionic sac can be explained reasonably
easily.
As is evident to any student studying the very young horse placenta, the
large allantoic vessels appear to lie free in a space which Enders & Liu (2000) showed to be the remains of the exocoelom. They also determined
the nature of collagen development of the basement membranes in these
young specimens.
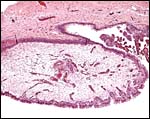
The hippomanes are yellowish/green pasty concentration products of fetal urine and contain large numbers of birefringent crystals (some of which are oxalates). The material is acellular and probably mostly composed of these crystals, protein and urinary detritus cells. The last kiang placenta was accompanied by one large 35 g flattened piece of hippomanes.
Equines have an initially large yolk sac that remains functional for a month and then regresses completely, leaving a tiny remnant in the cord insertion.
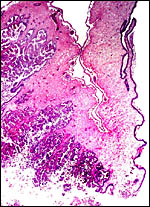
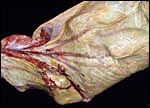
 |
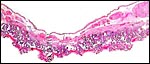
Section of the "allantoamnion", with allantois (vascularized) above, amnion (avascular) below. |
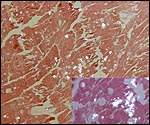 |
Hippomanes with birefringent crystals. Insert at higher magnification. |
The only "invasion" of uterine tissue occurs at the chorionic girdle with the development of temporary "endometrial cups". There is an intense inflammatory response, probably because of this infiltration. This is further discussed above and also in the section on immunology.
10)
Endometrium
Horse placentation is adeciduate (Ramsey, 1982). Thus, no true decidua
forms and endometrial glands are being maintained, albeit modified, during
the length of gestation. Importantly, there is extensive endometrial secretion
of various nutrients in the areolar regions (between the microcotyledons
(Wooding et al., 2000).
Vamanouchi et al. (1997) localized by hybridization studies of inhibin/activin
in different gestational ages that the beta A-subunit binds to the endometrial
epithelium and not to trophoblast. Thus, activin, rather than inhibin
is produced by the endometrium. Its role in gestation is as yet poorly
understood.
11)
Various features
The placentation of most equidae is extremely similar and, thus, hybridization
has been relatively often accomplished. The reader is also invited to
see the chapters on the Kiang and Grevy's zebra. Acker et al. (2001) have
described the morphology of equine embryos from days 17 to 40 after ovulation
and "staged" them uniformly. They serially sectioned the embryos
and found no anomalies, although there was a moderate degree of variation
of the first appearance of various structural features.
12) Endocrinology
An eminently readable general review of most endocrine parameters of equine
gestations was provided by Sharp (1999). It includes the seasonal aspects,
vernal transition and follicle maturation as well as pregnancy hormonal
changes and their measurements. The changes of specific gonadotropic cells
in the anterior pituitary during sexually active stages were defined by
Tortonese et al. (2001). Human chorionic gonadotropin (hCG) and a GnRH
analogue are used successfully in stimulating ovulation for artificial
insemination (Samper, 2001). What is interesting in equine gestation is
how the exact mechanism by which luteolysis (14 days after ovulation by
uterine prostaglandins) is prevented in successful gestations; it is still
under discussion. Sharp points out that some 20% failure rate exists at
this time. The exact (?) product from the early (unimplanted!) conceptus
that signals luteostasis, however, remains unknown. Because of the extensive
mobility of the conceptus during the first week and because of the luteolysis
that occurs when this is inhibited or restricted, a chemical signal appears
to be likely.
The production of eCG (equine chorionic gonadotropin) is clearly the most
unusual feature of the equine gestation. Allen & Stewart, in chapter
9 of McKinnon & Voss (1993) provided extensive details on the nature
of this hormone in equine gestation. eCG is definitely now known to be
produced by the trophoblast of the chorionic girdle. The cells can be
cultured in vitro and are then shown to produce the hormone. Serum
concentrations reach a peak around day 70 and then gradually fall. Nevertheless,
the continued presence of the hormone in circulation after abortion often
interferes with a new gestation. We assume also that this hormone may
be responsible for the enormous and most unusual stimulation of the fetal
gonads. It must be admitted, however, that this enlargement of gonads
occurs after the peak of eCG levels and that there is regression
of this interstitial component of the fetal gonads later in gestation.
In addition, it must also be said that no eCG has been found in fetal
fluids (Allen, 1982).
There is good evidence that this hormone is responsible for the recruitment
of additional stimulation and development of follicles in the maternal
ovaries, the so-called secondary corpora lutea. Alternatively, perhaps
waves of pituitary FSH stimulate maternal follicular growth with eCG's
luteotropic component causing their progesterone secretion. Many aspects
of equine hormonal regulation are still under active investigation as
some remain to be contradictory.
Allen et al. (2002b) studied eCG (formerly PMSG) and estradiol levels
in thoroughbred horses and ponies, and in their reciprocal embryo transfers
as well. The levels of eCG corresponded to the breed of the mare, rather
than to the transferred foal. The quantity of estrogens produced, however,
was greatest in the pregnancies with thoroughbred foals. The inference
is that this depended on the large gonads that are so markedly stimulated
in equine gestations. Progesterone levels also differed toward the end
of pregnancy.
Kirkpatrick et al. (1990) developed progesterone assays that permit one
to follow ovulation, and Czekala et al. (1990) evaluated estrone in urine
and serum of equids during pregnancy. Other special hormones produced
in equine gestation are equiline and equilenin; both are estrogens that
are presumably produced by aromatization of fetal androgen precursors
(Sharp, 1999). Allen & Stewart (2001) discussed the current status
of much of our understanding of equine endocrine aspects in pregnancy.
Marshall et al. (1999) identified numerous unconjugated steroids in larger
quantities within umbilical arterial blood (fetus-derived) than the venous
content. They discuss then pathways to C18 steroid and estrogen synthesis
which appears to differ in equine substantially from other mammals.
It should further be noted that the original hypothesis by Walton & Hammond (1938), namely that fetal size may be determined by endocrine
signals, has been disproved. They reciprocally crossed large Shire horses
and small Shetland ponies with the resulting fetuses being intermediate-sized
offspring. It was an ingenious experiment, but the recent study of embryo
transfers with similar-sized animals indicated that it is the uterine
size and the perfusion of uterus/placenta that regulate the fetal size,
rather than hormones (Allen et al., 1993; 2002b).
13)
Genetics
All domestic horses studied to date have had a chromosome number of 2n=64.
The presumed ancestor, the Przewalski's horse (Equus przewalskii)
has 2n=66, the donkeys have 2n=62 and zebras have 46, 44, and 32 chromosomes
respectively - from North to South (Benirschke et al., 1965; 1967; Ryder
et al., 1978). Hemiones have chromosomes in the 50s. An excellent study
of the banding patterns, telomeres and a proposal for standardization
of the donkey chromosomes comes from Raudsepp et al. (2000). Gadi &
Ryder (1983) described nucleolus-organizing regions in most equidae
and much more detailed modern genetic information was presented in chapters of the book by Bowling & Ruvinsky (2000).
Lear (2001) determined the chromosomal location of telomere sequence, and Milenkovic et al. (2002) localized 136 genes in the horse map. Lear et al. (1999) reported an autosomal trisomy (# 31) in a colt with significant anomalies and reviewed the other four trisomies (of chromosomes # 28, 23, 26, 30) described in horses.
Hybrids among nearly all equidae have been produced and most, if not all,
are sterile. The best known of these hybrids, the mule (and reciprocal
hinny), have 2n=63 and are also nearly always infertile. At least all
males have so far been azoospermic, and the very exceptional fertile female
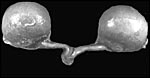
mule is noteworthy (Gray, 1972; Ryder et al., 1985; Benirschke & Ryder,
1985). These hybrids have been a "fertile" ground for research
on imprinting and epigenetic control of development, as their phenotypes
are so different (see for instance: Pennisi, 2001).
In recent years, gene localization is being studied in the horse, as for
instance the ![]() -globin
gene complex was assigned to chromosome 13 (Oakenfull et al., 1993). Preliminary
maps for chromosomes 1 and 10 were published by Kiguwa et al. (2000),
mitochondrial control regions were defined for the Mongolian wild horse
(Oakenfull & Ryder, 1998), and phylogenetic relations in equids were
established with the globin genes (Oakenfull & Clegg, 1998). The exploration
of this topic, however, has become too extensive for inclusion in this
short chapter. George & Ryder (1986) compared mtDNA of various equidae
to arrive at divergence rates. Holmes & Ellis (1999) investigated
the MHC class I antigen diversity of perissodactyla and concluded that
diversification in equids occurred after the split of horses from rhinoceroses.
-globin
gene complex was assigned to chromosome 13 (Oakenfull et al., 1993). Preliminary
maps for chromosomes 1 and 10 were published by Kiguwa et al. (2000),
mitochondrial control regions were defined for the Mongolian wild horse
(Oakenfull & Ryder, 1998), and phylogenetic relations in equids were
established with the globin genes (Oakenfull & Clegg, 1998). The exploration
of this topic, however, has become too extensive for inclusion in this
short chapter. George & Ryder (1986) compared mtDNA of various equidae
to arrive at divergence rates. Holmes & Ellis (1999) investigated
the MHC class I antigen diversity of perissodactyla and concluded that
diversification in equids occurred after the split of horses from rhinoceroses.
Chromosomal errors have been described primarily in sterile mares, with
63,X being the most common karyotype. In contrast to the human Turner's
syndrome (45,X) these mares are otherwise normal. Bowling & Hughes
(chapter 30 in McKinnon & Voss, 1992) reviewed all cytogenetic errors
then known as occurring in the horse. There are relatively few such chromosomal
errors, and studies of abortion specimens have not shown chromosomal abnormalities
to exist as the cause of abortion. Low-level mosaicism (XX/X) has been
described in three sterile or subfertile mares by Wieczorek et al., 2001).
Kent et al. (1986), reported XY sex reversal in the many horses and found
it to be a heritable condition with considerable phenotypic heterogeneity.
Mares with often marked masculinizing features possess XY chromosomes
and pedigrees show its inheritance.
Twinning is relatively common in horses (1-2%), but it is not often very
successful. Ginther (1982) stated that multiple ovulation occurs in Thoroughbreds
(19%), Quarter Horses (9%), and in Appaloosa (8%). Because of their precarious
outcome, most twins are eliminated by "s quashing" in early
gestation (day 30). Spontaneous abortion of one twin is also common. Whitwell
& Jeffcott (1975) classified the types of placentation in mares bearing
twins. Survivors may have had a fused placenta with anastomoses and are
then blood chimeric (Podliachuk et al., 1974). No good evidence of freemartinism
in the females with male blood admixture had been forthcoming according
to these authors. Nevertheless, at least two cases of freemartinism in
horses due to chimerism and placental anastomoses were reviewed earlier
(Benirschke, 1970). Furthermore, several cases of acardiac twinning have
been recorded (see section of pathology).
 |
G-banded karyotype of male domestic horse (Courtesy: Teri Lear) |
 |
Exceptional mule ("Krause") with offspring ("Blue Moon"). |
14)
Immunology
The immunologic aspect of equine gestation has fascinated many investigators.
While most of the fetal/maternal relation is epithelio-chorial and gives
no reason for an immune response, the unusual development of "endometrial
cups" at the chorionic girdle is different. Here, trophoblast invades
the endometrium, destroys its epithelium and elicits an intense, yet transitory,
lymphocellular and plasmacytic reaction whose raison d'être is still
unclear, especially since it is transitory. Sharp (1999) suggested it
to represent the "testing of the waters".
The major histocompatibility antigen complex (MHC) has been studied, especially
often in relation to the invasive chorionic girdle cells and the attending
inflammation (for instance, Baker et al., 2001 and numerous others cited
there). This is a major, ongoing study from which much will be learned.
It is helped by the availability of models such as the mule and the donkey-in-horse
exchanges. So far, no genetic differences in horse and donkey MHC structure
have been identified. Bacon et al. (2002) described MHC alloantigens in
horse trophoblast. Hedrick et al. (1999) defined the MHC type II antigens
in 14 Przewalski's horses and found very low variability. Baker et al.
(1999) were surprised at findings in interspecific pregnancies (mares
carrying mules). There was failure to down-regulate the usual cellular
immune response. Transplanted girdle trophoblast (outside the uterus)
differentiated normally and evaded maternal immune response (Adams & Antczak, 2001). Later, Baker et al. (2001) identified five donkey alloantigens
of the MHC type I alleles by the use of interspecific equine pregnancies.
They concluded that it was the relatively close relationship of horse
and donkey that allowed the frequent success of hybrid pregnancies, despite
the presence of alloantigens and focally invasive placentation, while
in some other (cited) attempts a hybridization, most implantations fail.
15) Pathological features
Numerous pathological features of the reproductive organs have been summarized
by McEntee (1990). They include endometrial atrophy, mucometra, pyometra,
occasional tumors of the endometrium and some congenital anomalies. More
important, some of these conditions have been the causes of reproductive
failure and abortion (Platt, 1973a,b). Prickett (1970) reviewed the causes
of abortion and placental lesions in mares. He considered that premature
placental detachment ("red bag") was a prevalent cause of fetal
demise and listed fetal diarrhea (meconium discharge) as an important
event that may occur at any time in pregnancy. An important survey of
a large number of spontaneous abortions and stillbirth at Newmarket has
been produced by Smith et al. (2003). As was suggested earlier by Hong
(v.i.), an excessively long hypertwisted (torsion) umbilical
cord played a major role (38.8%). It is interesting to read that the cord
may be so twisted that it obstructs the urine flow in the allantoic duct.
Certainly human abortions and growth restriction in utero are frequent
causes of abortion and stillbirth, but they generally are due restricted
venous return from the placenta. Other major causes are ascending infection,
usually most pronounced at the cervical star, herpes infection and sundry
other inflammatory lesions. They refer to the apparently greater frequency
of leptospirosis in Kentucky than occurring in the UK (v.i.).
Infections (ascending or hematogenous) were other listed causes of abortion
with a variety of organisms the culprits. A more detailed examination
of the causes of abortion and stillbirths is that by Giles et al. (1993)
from their extensive experience in Kentucky. Bacterial infection was found
to be the primary cause of fetal demise, followed by viral infection (equine
herpes virus) and fungal infection next. "Placentitis" without
recovered organisms was another major cause of demise. The most commonly
recovered bacteria were streptococci, coliforms and leptospira. Other
normal, commensal, oral organisms were also identified. In a Przewalski's
horse at the San Diego Zoo we observed abortion in the first trimester
of a foal due to placentitis and cultured Bacillus mycoides. The
origin of this organism remained obscure. Interestingly, only some portions
of the placenta showed intense inflammation, other regions remained normal.
Patterson-Kane et al. (2002) found Rhodococcus equi as a cause
of abortion in a thoroughbred mare. Leptospirosis and other organisms
were identified by Donahue & Williams (2000) as causes of mare abortions.
In reviewing the histopathology of many different types of intrauterine
infection it is evident that the morphology differs from the common human
infection, "chorioamnionitis". The inflammation is much more
focal (other regions of the placenta may be entirely normal), is often
associated with necrosis (obviously dependent on the organism) and the
umbilical vessels are usually not inflamed, despite the fact that peripheral
funisitis is common. One such abortion case at 9 months due to Leptospira
pomona was supplied to me by Dr. P. Pesavento of Davis, CA. The organisms
were identified with certainty only in the fetal kidneys, where they were
abundant. There was much coagulative necrosis and associated inflammation
in this placenta from a warmblood living on a pasture next to a cattle
ranch. Leptospirosis abortion has a huge bibliography and is not an uncommon
condition in equines. Hodgin et al. (1989) reported 4 cases of late abortion;
Donahue et al. (1992), Poonacha et al. (1993), Giles et al. (1993) and
Donahue et al. (1995) made huge contributions to our understanding this
condition with their reports from Kentucky. Kinde et al. (1996) reported
its occurrence following a flooding incident at Davis, and numerous other
papers could be cited. The diagnosis is generally made by serology (Williams
et al., 1994), Warthin Starry stains of kidneys, or by DNA fragment analysis
(Lucchesi et al., 2002).
A major outbreak of abortion on Kentucky horse farms occurred in 2001/2002
with hundreds of aborted or stillborn foals. It is now known as MRLS (Mare
Reproductive Loss Syndrome) and the symposium has
now been published (Powell et al., 2003). Placental infection was identified
and commensal organisms were also frequent offenders in some of the aborted
foals or of weak neonates. One of these organisms was Actinobacillus sp., a common oral species with significant cytolethality (DiRienzo et
al., 2002). Stewart et al. (2002) found it to cause bacteremia in foals.
Perhaps this and perhaps other organisms were the prime agents during
this epidemic. Whether the "epidemic" was causally related to
the contemporaneous population explosion of Eastern Tent Caterpillars
(ETC) in the region has not been completely resolved. The placentas were
often edematous. "Contracted foals" (arthrogryposis) and other
congenital anomalies were found in 10% of cases of abortions and stillbirths.
In general, however, ascending infection from the lower genital tract
was the most common mode of infection, but hematogenous spread also occurred
(Harrison et al., 2002). In addition, encephalitis due to Actinobacillus
infection was reported in three horses by Sebastian et al. (2002). Another
thought of the cause of this unprecedented mortality is the ingestion
or acquisition of cyanides from food sources, perhaps by inhalation (Burns,
personal communication, 2002). Mandelonitrile (with cyanide production)
from abundant black cherry tree leaves and ingested by ETC may have caused
capillary damage that results in the syndrome. It is currently under active
study.
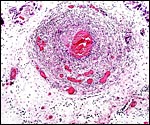
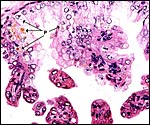
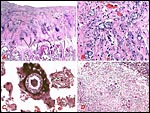 |
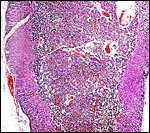
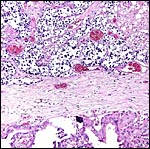
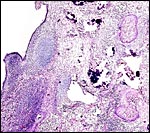
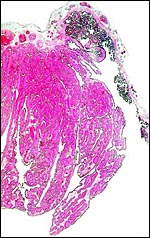
Composite of transplacental infection with Coccidioides immitis. A: chorion with granulomas; B: spherules in chorion; C: spherule in placental villus; D: fetal lung with numerous granulomas. |
In
addition to these reasons for abortion, an excessively long umbilical
cord often caused fetal demise by "strangulation". This has
been alluded to in the discussion on umbilical cord above and is also
the topic of the publications by Vandeplassche & Lauwers (1986), and
that by Hong et al. (1993). The latter authors found the normal cord length
to be 52 cm; 4.5% of 1,211 abortion cases were due to excessively long
cords with torsion and they were 72 cm long. The large survey of abortions
and stillbirths by Smith et al. (2003) makes the same observations. What
remains to be elucidated is why this is so relatively common in horses,
as compared to its rare occurrence in most other species.
Endometritis is not infrequent and may be acquired by mating with an infected
stallion (Platt et al., 1978), as well as easily created experimentally.
Another commonly experienced condition that threatens gestations is "fescue
toxicosis". Tall fescue (tough meadow grasses) is often heavily infected
with fungi that produce ergot alkaloids and, when ingested, may lead to
impaired placental performance and insufficiency with abortion (Hoveland,
1993; others). Most recently, West Nile fever virus infection was diagnosed
in 30 horses of Florida (Wamsley et al., 2002) as well as in other Italian
and American horses (Del Piero et al., 2002).
Equine specialists often speak of the "red bag" with which they
mean a placenta presenting as an unusually dark red organ and they infer
from this appearance that it had abrupted in utero, perhaps causing fetal
demise.
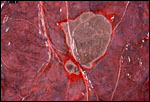
Acardiac fetuses are the result of reversal of circulation in twins with
placental anastomoses. They are not uncommon in human pregnancies where
they generally represent one of monozygotic twins in which the normal
twin "nourishes" the acardiac by reversal of blood flow. In
horses, only some 4-5 cases have been described; more may occur but are
overlooked. Höfliger (1974) reviewed some cases, and Crossman &Dickens
(1974) illustrated such a 500 g "monster". We have seen a similar
so-called acardiac amorphous ("globosus"), similarly attached
to the insertion of the umbilical cord of a placenta. It was 12 cm in
size, was also attached to the cord in its allantoic portion, contained
blood and had a calcified shell. The soft tissue inside had fat, connective
tissue and blood vessels. It has been suggested to us that this may not
be an acardiac fetus but, rather, that this is the remains of a yolk sac.
That is possible and bears future studies. In
contrast to human acardiacs, where the karyotype is generally that of
the co-twin and normal, no genetic studies have been reported in equine
acardiacs. They would be of interest, as placental anastomoses exist occasionally
between equine fraternal twins as well.
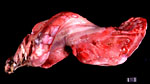
In addition to the acardiac twin, we have described a placental teratoma (Gurfield & Benirschke, 2003). Interestingly, the mare remained well later, thus precluding metastases from an ovarian teratoma. Most of the tissue had the appearance of dysgerminoma, but more mature elements (skin, thyroid, cartilage, etc.) were also found. It may therefore be of additional interest that we have identified two cases of benign cystic teratoma in the membranes of kiang placentas (see chapter on Kiang). Since that publication, another placental teratocarcinoma with possible metastases to the foal was described by Allison et al. (2004). The foal died in 2½ months from metastases.
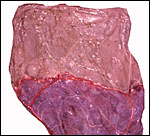
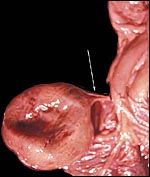
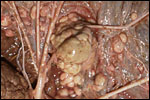
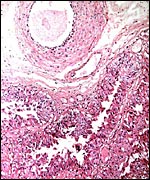
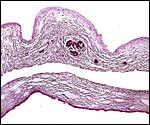
Griner (1983) summarized a variety of pathologic features that he identified in exotic equidae of the San Diego Zoo. Trauma, neonatal deaths from omphalitis and intestinal helminths were the principal reasons for mortality. The next pictures are from a "papillary region" in the surface of the placenta that was initially interpreted as being squamous metaplasia. Higher magnification shows no desmosomes and the hypertrophied cells are continuous with the villous trophoblast. We have seen only one such lesion and believe that it represents a benign proliferation of trophoblast.
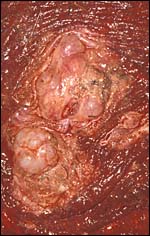 |
"Adenomatous hyperplasia of allantois" in a somewhat weak horse neonate. (Courtesy, Manu Sebastian, Lexington, KY). |
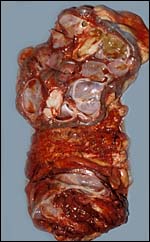 |
Another example of allantoic adenoma. |
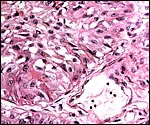
The next lesion of a horse placenta was sent to me by Dr. Berrington of Langley, British Columbia. The foal was normal and survived; the lesions come from the lesser horn and were described as "abundant, scattered fatty masses" attached to the maternal surface. Despite the vast experience of the breeder and pathologist, such lesions had never been seen and the question was raised whether this may be the equivalent of molar degeneration of human placentas. A few of the lesions were quite large and grape-like. In my opinion the lesions are more like what in human pathology we refer to as "chorangiomatosis", a proliferation of fetal villus capillaries and occurring in chronic hypoxic conditions as well as for no apparent reason.
Cowell & Tyler (2001) provided a detailed review of the hematology and cytology of numerous fluids and tissues. The relationship of fetal size to maternal size and the "supply line" has been examined several times, but most decisively by Allen et al. (2002a). They found that ponies had a 325 day gestation, while the thoroughbreds had a length of 339 days. To further understand the finer reproductive regulations, embryo transfer experiments were conducted. These were also designed to verify the original observations by Walton & Hammond of crossbreeding Shire horse with Shetland ponies (1938; see above). When embryo transfers were performed between these two breeds then the gestational length was intermediate, as were the foals' weights. The ponies in thoroughbred mares were significantly heavier and correlated with weights of mare and placental sizes. Moreover, the density of microcotyledons was greater in the thoroughbreds, irrespective of the foal they carried. Thus, the placental contact area, rather than genetic background of the horses is important. Endocrine findings were more complex (Allen et al., 2002b, see section 12 above).
Wooding & Morgan (1988) defined the sites for calcium and glucose exchange in the areolar regions of the placenta. In subsequent studies, Wooding et al. (2000) showed iron transport to be achieved by the uteroferrin, a protein secreted by the endometrium in response to progesterone (Groothuis et al., 1997). Its presence can be identified in the microvesicular transport system, different from the facilitated transport of calcium. This protein, as well as many other components of uterine secretions at various cycle days were better defined by Zavy et al. (1982).
Ridderstrale et al. (1997) studied the presence of carbonic anhydrase at the interface in a variety of species. There was relatively little activity in horse placentas.
17)
Other resources
Strains of fibroblast cells of most wild and domestic equidae are contained
in the "Frozen Zoo" at CRES, the research division of the Zoological
Society of San Diego. They can be made available on request to Dr. Oliver
Ryder at oryder@ucsd.edu.
Thway et al. (2001) at Kansas Medical Center produced immortalized cell
lines from girdle cells that exhibited a variety of characteristics, well-studied
by these investigators; they were immortalized prior to their eCG productive
ability, however.
18)
Other remarks - What additional Information is needed?
As has been alluded to above, it would be of interest to know the lengths
of umbilical cords in different breeds of horses. Also, the mechanism
leading to the marked gonadal stimulation of the fetuses is of future
interest.
The first successful cloning of equids was reported by Woods et al. (2003).
A mule (cells from a 45 day embryo) was cloned into quarterhorse oocytes
and the first live cloned equine foal was thus achieved in Iowa. The report
suggests that births of additional horses can be anticipated. What may
be of importance in this experiment is that the medium for culture of
the cloned oocytes occurred in a 3-fold calcium concentration and that
embryonic cells were employed. In August, 2003, a second normal foal was
cloned in Italy (Galli, 2003). This report also presents new methodology
of handling the newly constituted ovum with much improved success a sequel.
The first successful cloning of equids was reported by Woods et al. (2003).
A mule (cells from a 45 day embryo) was cloned into quarterhorse oocytes
and the first live cloned equine foal was thus achieved in Iowa. The report
suggests that births of additional horses can be anticipated. What may
be of importance in this experiment is that the medium for culture of
the cloned oocytes occurred in a 3-fold calcium concentration and that
embryonic cells were employed.
19) Acknowledgement
We are grateful to Teri Lear in Lexington for the banded horse karyotype.
Special thanks also to Katherine Whitwell of Cambridge, UK for numerous
suggestions and corrections. Dr. Allan J. Berrington of Langley, BC kindly
submitted an abnormal placenta.
References
Acker, D.A., Curran, S., Bersu, E.T. and Ginther, O.J.: Morphologic stages
of the equine embryo proper on days 17 to 40 after ovulation. Amer. J.
Vet. Res. 62:1358-1364, 2001.
Adams, A.P. and Antczak, D.F.: Ectopic transplantation of equine invasive trophoblast. Biol. Reprod. 64:753-763, 2001.
Allen, W.R.: Immunological aspects of the endometrial cup reaction and the effect of xenogeneic pregnancy in horses and donkeys. J. Reprod. Fertil. Suppl. 31:57-94, 1982.
Allen, W.R., Skidmore, J.A., Stewart, F. and Antzak, D.F.: Effects of fetal genotype and uterine environment on placental development in equids. J. Reprod. Fertil. 97:55-60, 1993.
Allen, W.R. and Stewart, F.: Equine placentation. Reprod. Fertil. Devel. 13:623-634, 2001.
Allen, W.R., Wilsher, S., Turnbull, C., Stewart, F., Ousey, J., Rossdale, P.D. and Fowden, A.L.: Influence of maternal size on placental, fetal and postnatal growth in the horse. I. Development in utero. Reproduction 123:445-453, 2002a.
Allen, W.R., Wilsher, S., Stewart, F., Ousey, J., Ousey, J. and Fowden, A.L.: The influence of maternal size on placental, fetal and postnatal growth in the horse. II. Endocrinology of pregnancy. J. Endocrinol. 172:237-246, 2002b.
Allen,
W.R., Wilsher, S. and Morris, L.: Profound influences of uterine genotype
on placental differentiation in equids. Placenta 23 (7): abstract P3,
2002c.
Allison, N., Moeller, R.B.Jr. and Duncan, R.: Placental teratocarcinoma in a mare with possible metastasis to the foal. J. Vet. Diagn. Invest. 16:160-163, 2004.
Amoroso, E.C.: Placentation. Chapter 15, pp.127-311, In, Marshall's Physiology of Reproduction, V.II, A.S. Parkes, ed. Second Edition. Little, Brown & Co. Boston, 1961.
Bacon,
S.J., Ellis, S.A. and Antczak, D.F.: Control of expression of major histocompatibility
complex genes in horse trophoblast. Biol. Reprod. 66:1612-1620, 2002.
Baker, J.M., Bamford, A.I. and Antczak, D.F.: Modulation of allospecific
CTL responses during pregnancy in equids: an immunological barrier to
interspecies matins? J. Immunol. 162:4496-4501, 1999.
Baker, J.M., Stidworthy, M., Gull, T., Novak, J., Miller, J.M. and Antczak, D.F.: Conservation of recognition of antibody and T-cell-defined alloantigens between species of equids. Reprod. Fertil. Dev. 13:635-645, 2001.
Benirschke, K.: Spontaneous chimerism in mammals. A critical review. Current Topics in Pathol. 51:1-61, 1970.
Benirschke, K.: Obstetrically important lesions of the umbilical cord. J. Reprod. Med. 39:262-272, 1994.
Benirschke, K.: Fetal consequences of amniotic fluid meconium. Contemporary Obstetrics & Gynecology 46:76-83, 2001.
Benirschke,
K., Malouf, N., Low, R.L. and Heck, H.: Chromosome complement: Difference between Equus caballus and Equus przewalskii,
Poliakoff. Science 148:382-383, 1965.
Benirschke, K. and Malouf, N.: Chromosome studies of Equidae. In: Equus,
Vol. 1 & 2. H. Dathe, ed. Presented at the Second International Symposium
on the Przewalski's Horse, Tierpark Berlin, 1965, pp. 253-284, 1967.
Benirschke, K. and Ryder, O.A.: Genetic aspects of equids with particular reference to their hybrids. Equine Vet. J. Suppl. 3:1-10, 1985.
Bergfelt, D.R., Gastal, E.L. and Ginther, O.J.: Response of estradiol and inhibin to experimentally reduced luteinizing hormone during follicle deviation in mares. Biol. Reprod. 65:426-432, 2001.
Betteridge,
K.J, Eaglesome, M.D., Mitchell, D., Flood, P.F. and Beriault, R.: Development
of horse embryos up to twenty-two days after ovulation: observations on
fresh specimens. J. Anat. 135:191-209, 1982.
Bowling, A.T. and Ruvinsky, A.: The Genetics of the Horse. CABI Publishing, 2000.
Caslick, E.A.: Notes on equine placentae. Proc. Kentucky Vet. Med. Ass. Pp. 54-56, 1932.
Cothran, E.G., Santos, S.A., Mazza, M.C.M., Lear, T. and Sereno, J.R.B.: Genetics of the Pantaneiro horse of the Pantanal region of Brazil. Genet. Molec. Biol. 21:343-349, 1998.
Cowell, R.L. and Tyler, R.D.: Diagnostic Cytology and Hematology of the Horse, 2nd ed. Mosby, Elsevier Science, 2001.
Crossman, P.J. and Dickens, P.S.E.M.: Amorphus globosus in the mare. Vet. Rec. 95:22, 1974.
Czekala, N.M., Kasman, L.H., Allen, J., Oosterhuis, J. and Lasley, B.L.: Urinary steroid evaluations to monitor ovarian function in exotic ungulates. VI. Pregnancy detection in exotic equidae. Zoo Biol. 9:43-46, 1990.
Del Piero, F., Cantile, C., Di Guardo, G., Bioletti, B. and Arispici, M.: Natural West Nile flavovirus infection in horses in the USA and Italy. Vet. Pathol. 39:630 (abstract 69), 2002.
DiRienzo,
J.M., Song, M., Wan, L.S. and Ellen, R.P.: Kinetics of KB and HEp-2 cell
responses to an invasive, cytolethal distending toxin-producing strain
of Actinobacillus actinomycetemcomitans. Oral Microbiol.
Immunol. 17:245-251, 2002.
Donahue, J.M., Smith, B.J., Donahoe, J.K., Rigsby, C.L., Tramontin, R.R.,
Poonacha, K.B. and Wilson, M.A.: Prevalence and serovars of leptospira
involved in equine abortions in central Kentucky during the 1990 foaling
season. J. Vet. Diagn. Invest. 4:279-284, 1992.
Donahue, J.M., Smith, B.J., Poonacha, K.B., Donahoe, J.K. and Rigsby, C.L. Prevalence and serovars of leptospira involved in equine abortions in central Kentucky during the 1991-1993 foaling seasons. J. Vet. Diagn. Invest. 7:87-91, 1995.
Donahue, J.M. and Williams, N.M.: Emergent causes of placentitis and abortion. Vet. Clin. North Amer. Equine Pract. 16:443-456, 2000.
Enders, A.C. and Liu, I.K.M.: Lodgement of the equine blastocyst in the uterus from fixation through endometrial cup formation. J. Reprod. Fertil. Suppl. 44:427-438, 1991a.
Enders, A.C. and Liu, I.K.M.: Trophoblast-uterine interactions during equine chorionic girdle cell maturation, migration, and transformation. Amer. J. Anat. 192:366-381, 1991b.
Enders, A.C. and Liu, I.K.M.: A unique exocoelom-like space during early pregnancy in the horse. Placenta 21:575-583, 2000.
Enders, A.C., Meadows, S., Stewart, F. and Allen, W.R.: Failure of endometrial cup development in the donkey-in-horse model of equine abortion. J. Anat. 188:575-589, 1996.
Enders, A.C., Jones, C.J., Lantz, K.C., Schlafke, S. and Liu, I.K.M.: Simultaneous exocrine and endocrine secretion: trophoblast and glands of the endometrial cups. J. Reprod. Fertil. Suppl. 56:615-625, 2000.
Gadi,
I.K. and Ryder, O.A.: Distribution of silver-stained nucleolus-organizing
regions in the chromosomes of the equidae. Genetica 62:109-116, 1983.
Galli, C., Lagutina, I., Crotti, G., Colleoni, S., Turini, P., Ponderato,
N., Duchi, R. and Lazzari, G.: A cloned horse born to its dam twin. Nature
424:635, 2003.
Giles, R.C., Donahue, J.M., Hong, C.B., Tuttle, P.A., Petrites-Murphy, M.B., Poonacha, K.B., Roberts, A.W., Tramontin, R.R., Smith, B. and Swerczek, T.W.: Causes of abortion, stillbirth, and perinatal death in horses: 3,527 cases (1986-1991). J. Amer. Vet. Med. Assoc. 203:1170-1175, 1993.
George, M. and Ryder, O.A.: Mitochondrial DNA evolution in the genus Equus. Mol. Biol. Evol. 3:535-546, 1986.
Giles, R.C., Donahue, J.M., Hong, C.B., Turtle, P.A., Petrites-Murphy, M.B., Poonacha, K.B., Roberts, A.W., Tramontin, R.R., Smith, B. and Swerczek, T.W.: Causes of abortion, stillbirth, and perinatal death in horses: 3,527 cases (1986-1991). JAVMA 203:1170-1175, 1993.
Ginther, O.J.: Twinning in mares: a review of recent studies. Equine Vet. Sci.2:127-135, 1982.
Ginther, O.J.: Dynamic physical interactions between the equine embryo and uterus. Equine Vet. J. Suppl. 3:41-47, 1985.
Ginther, O.J., Beg, M.A., Bergfelt, D.R., Donadeu, F.X. and Kot, K.: Follicle selection in monovular species. Biol. Reprod. 65:638-647, 2001.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Groothuis,
P.G., Blair, R.M., Simmen, R.C., Vallet, J.L., Grieger, D.M. and Davis,
D.L.: Uterine response to progesterone in prepubertal gilts. J. Reprod.
Fertil. 110:237-243, 1997.
Gurfield, N. and Benirschke, K.: Equine placental teratoma. Vet. Path. 40:586-588, 2003.
Harrison, L., Giles, R., Williams, N., Donahue, J., Hong, C.B., Poonacha, K.R., Bolin, D., Jackson, C., Roberts, J., Sebastian, M., Tramontin, R., Smith, R., Vickers, M., Sells, S. and Smith, B.: Mare reproductive loss syndrome: pathologic observations. Vet. Pathol. 39:630 (abstract 72), 2002.
Hedrick,
P.W., Parker, K.M., Miller, E.L. and Miller, P.S.: Major histocompatibility
complex variation in the endangered Przewalski's horse. Genetics 152:1701-1710,
1999.
Hodgin, E.C., Miller, D.A. and Lozano, F.: Leptospira abortion in horses.
J. Vet. Diagn. Invest. 1:283-287, 1989.
Höfliger, H.: Beitrag zur Kenntnis der Akardier. Schweiz. Arch. Tierheilk. 116:629-644, 1974.
Holmes, E.C. and Ellis, S.A.: Evolutionary history of MHC class I genes in the mammalian order Perissodactyla. J. Mol. Evol. 49:316-324, 1999.
Hong,
C.B., Donahue, J.M., Giles, R.C., Petrites-Murphy, M.B., Poonacha, K.B.,
Roberts, A.W., Smith, B.J., Tramontin, R.R., Tuttle, P.A. and Swerczek,
T.W.: Equine abortion and stillbirth in central Kentucky during 1988 and
1989 foaling seasons. J. Vet. Diagn. Invest. 5:560-566, 1993a.
Hong, C.B., Donahue, J.M., Giles, R.C., Petrites-Murphy, M.B., Poonacha,
K.B., Tramontin, R.R., Tuttle, P.A. and Swerczek, T.W.: Adenomatous hyperplasia
of equine allantoic epithelium. Vet. Pathol. 30:171-175, 1993b.
Hoveland, C.S.: Importance and economic significance of the Acremonium endophytes to performance of animals and grass plants. Agricult. Ecosyst. Environm. 44:33, 1993.
Jones,
C.J., Wooding, F.B., Abd-Elnaeim, M.M., Leiser, R., Dantzer, V. and Stoddart,
R.W.: Glycosylation in the near-term epithelio-chorial placenta of the
horse, donkey and camel: a comparative study of interbreeding and non-interbreeding
species. J. Reprod. Fertil. 118:397-405, 2000.
Kent, M.G., Shoffner, R.N., Buon, L. and Weber, A.F.: XY sex-reversal
syndrome in the domestic horse. Cytogenet. Cell Genet. 42:8-18, 1986.
Kiguwa,
S.L., Hextall, P., Smith, A.L., Critcher, R., Swinburne, J., Millon, L.,
Binns, M.M., Goodfellow, P.N., McCarthy, L.C., Farr, C.J. and Oakenfull,
E.A.: A horse whole-genome-radiation hybrid panel: chromosome 1 and 10
preliminary maps. Mamm. Genome 11:803-805, 2000.
Kinde, H., Hietala, S.K., Bolin, C.A. and Dowe, J.T.: Leptospiral abortion
in horses following a flooding incident. Equine Vet. J. 28:327-330, 1996.
Kirkpatrick, J.F., Lasley, B.L. and Shideler, S.E.: Urinary steroid evaluations to monitor ovarian function in exotic ungulates. VII. Urinary progesterone metabolites in the equidae assessed by immunoassay. Zoo Biol. 9:341-348, 1990.
Klonisch, T., Ryan, P.L., Yamashiro, S. and Porter, D.G.: Partial complementary deoxyribonucleic acid cloning of equine relaxin messenger ribonucleic acid, and its localization within the equine placenta. Biol. Reprod. 52:1307-1315, 1995.
Lear, T.L.: Chromosomal distribution of the telomere sequence (TTAGGG) n in the equidae. Cytogenet. Cell Genet. 93:127-130, 2001.
Lear, T.L., Cox, J.H. and Kennedy, G.A.: Autosomal Trisomy in a Thoroughbred colt: 65,XY,+31. Equine Vet. J. 31:85-88, 1999.
Lucchesi, P.M., Parma, A.E. and Arroyo, G.H.: Serovar distribution of
a DNA sequence involved in the antigenic relationship between Leptospira
and equine cornea. BMC Microbiol. 2002;2(1):3. Epub. 2002 Feb. 13.
Macdonald, A.A., Chavatte, P. and Fowden, A.L.: Scanning electron microscopy of the microcotyledonary placenta of the horse (Equus caballus) in the latter half of gestation. Placenta 21:565-574, 2000.
Marshall, D.E., Gower, D.B., Silver, M., Fowden, A. and Houghton, E.: Cannulation in situ of equine umbilicus. Identification by gas chromatography-mass spectrometry (GC-MS) of differences in steroid content between arterial and venous supplies to and from the placental surface. J. Steroid Biochem. Mol. Biol. 68:219-228, 1999.
McEntee,
K.: Reproductive Pathology of Domestic Mammals. Academic Press, San Diego,
1990.
McEntee, M., Brown, T. and McEntee, K.: Adenomatous dysplasia of the equine
allantois. Vet. Pathol. 25:387-389, 1988.
McKinnon, A.O. and Voss, J.L.: Equine Reproduction. Lea & Febiger, Philadelphia, 1992.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Oakenfull,
E.A., Buckle, V.J. and Clegg, J.B.: Localization of the horse (Equus
caballus) ![]() -globin
gene complex to chromosome 13 by fluorescence in situ hybridization. Cytogenet.
Cell Genet. 62:136-138, 1993.
-globin
gene complex to chromosome 13 by fluorescence in situ hybridization. Cytogenet.
Cell Genet. 62:136-138, 1993.
Oakenfull, E.A. and Clegg, J.B.: Phylogenetic relationships within the genus Equus and the evolution of alpha and theta globin genes. J. Mol. Evol. 47:772-783, 1998.
Oakenfull, E.A. and Ryder, O.A.: Mitochondrial control region and 12S rRNA variation in Prezewalskii horse (Equus przewalskii). Anim. Genet. 29:456-459, 1998.
Patterson-Kane, J.C., Donahue, J.M. and Harrison, L.R.: Placentitis, fetal pneumonia, and abortion due to Rhodococcus equi infection in a thoroughbred. J. Vet. Diagn. Invest. 14:157-159, 2002.
Platt, H.: Etiological aspects of perinatal mortality in the thoroughbred. Equine Vet. J. 5:116-120, 1973a.
Platt, H.: Infection of the horse fetus. J. Reprod. Fertil. Suppl. 23:605-610, 1975b.
Platt, H., Atherton, J.G. and Simpson, D.J.: The experimental infection of ponies with contagious equine metritis. Equine Vet. J. 10:153-159, 1978.
Pennisi, E: Epigenetics. Behind the scenes of gene expression. Science 203:1064-1067, 2001.
Podliachouk,
L., Vandeplassche, M. and Bouters, R.: Gestation gémellaire, chimérisme
et freemartinisme chez le cheval. Acta Zool. Pathol. Antv. 58:13-28, 1974.
Poonacha, K.B., Donahue, J.M., Giles, R.C., Hong, C.B., Petrites-Murphy,
M.B., Smith, B.J., Swerczek, T.W., Tramontin, R.R. and Tuttle, P.A.: Leptospirosis
in equine fetuses, stillborn foals, and placentas. Vet. Pathol. 30:362-369,
1993.
Powell,
D.G., Troppman, A. and Tobin, T., eds.: Proceedings of the First Workshop
on Mare Reproductive Loss Syndrome. Kentucky Agricultural Experiment Station,
Lexington, KY, 2003.
Powell, D.G., Furry, D. and Hale, G.: Proceedings of a Workshop on the Equine Placenta. Kentucky Agricultural Experiment Station, University of Kentucky , M. Gluck Equine Research Center , Lexington , Kentuck 2004. (The volume is available upon request.).
Prickett,
M.E.: Abortion and placental lesions in the mare. JAVMA 157:1465-1470,
1970.
Ramsey, E.M.: The Placenta. Human and Animal. Praeger Publishers, New York, 1982.
Raudsepp, T., Christensen, K. and Chowdhary, B.P.: Cytogenetics of donkey chromosomes: nomenclature proposal based on GTG-banded chromosomes and depiction of NORs and telomeric sites. Chromosome Res. 8:659-670, 2000.
Ridderstrale, Y., Persson, E., Dantzer, V. and Leiser, R.: Carbonic anhydrase activity in different placenta types: a comparative study of pig, horse, cow, mink, rat, and human.Microsc. Res. Tech. 38:115-124, 1997.
Ryan, P., Bennet-Wimbush, Vaala, W.E. and Bagner, C.A.: Relaxin as a biochemical marker of placental insufficiency in the horse: a review. Pferdeheilkunde 15:622-628, 1999.
Ryder, O.A., Epel, N.C. and Benirschke, K.: Chromosome banding studies of the equidae. Cytogenet. Cell Genet. 20:323-350, 1978.
Ryder, O.A., Chemnick, L.G., Bowling, A.T. and Benirschke, K.: Male mule foal qualifies as the offspring of a female mule and jack donkey. J. Heredity 76:379-381, 1985.
Samper, J.C.: Equine Breeding Management and Artificial Insemination. Mosby, Elsevier Science, 2000.
Samper, J.C.: Management and fertility of mares bred with frozen semen. Anim. Reprod. Sci. 68:219-228, 2001.
Sebastian, M., Giles, R., Donahue, J., Sells, S., Tramontin, R., Poonacha, K.B., Roberts, J., Harrison, L., Seahorn, T., Sprayberry, K. and Bernard, W.: Encephalitis due to Actinobacillus species in three adult horses. Vet Pathol. 39:630 (abstract 70), 2002.
Sharp,
D.C.: Horses. In Encyclopedia of Reproduction, Vol. 2, pp 680-693, E.
Knobil & eds., Academic Press, San Diego, 1999.
Shivaprasad, H.L., Sundberg, J.P., McEntee, K., Gordon, L., Johnstone,
A.C., Lombardo de Barros, C.S. and Hoffman, R.L.: Cystic adenomatous hyperplasia
of the equine allantois: a report of eight cases. J. Vet. Diagn. Invest.
6:107-110, 1994.
Simpson,
G.G.: Horses. Oxford Press, 1951.
Smith, K.C., Blunden, A.S., Whitwell, K.E., Dunn, K.A. and Wales, A.D.:
A survey of equine abortion, stillbirth and neonatal death in the UK from
1988 to 1997. Equine Vet. J. 35:496-501, 2003.
Snider, W.: Short umbilical cords. J. Perinatol. 17:327-329, 1997.
Steven, D.H.: Placentation in the mare. J. Reprod. Fertil. Suppl. 31:41-55, 1982.
Stewart, A.J., Hinchcliff, K.W., Saville, W.J., Jose-Cunilleras, E., Hardy, J., Kohn, C.W., Reed, S.M. and Kowalski, J.J.: Actinobacillus sp. bacteremia in foals: clinical signs and prognosis. J. Vet. Intern. Med. 16:464-471, 2002.
Thenius, E. and Hofer, H.: Stammesgeschichte der Säugetiere. Springer-Verlag, Berlin, 1960.
Thway, T.M., Clay, C.M., Maher, J.K., Reed, D.K., McDowell, K.J., Antczak, D.F., Eckert, R.L., Nilson, J.H. and Wolfe, M.W.: Immortalization of equine trophoblast cell lines of chorionic girdle cell lineage by simian-40 large T antigen. J. Endocrinol. 171:45-55, 2001.
Tortonese, D.J., Gregory, S.J., Eagle, R.C., Sneddon, C.L., Young, C.L. and Townsend, J.: The equine hypophysis: a gland for all seasons. Reprod. Fertil. Dev. 13:3591-597, 2001.
Vamanouchi, K., Hirasawa, K., Hasegawa, T., Ikeda, A., Chang, K.T., Matsuyama, S., Nishihara, M., Miyazawa, K., Sawasaki, T., Tojo, H., Tachi, C. and Takahashi, M.: Equine inhibin/activin beta A-subunit mRNA is expressed in the endometrial gland, but not in the trophoblast, during pregnancy. Mol. Reprod. Dev. 47:363-369, 19997.
Vandeplassche, M. and Lauwers, H.: The twisted umbilical cord: an expression of kinesis of the equine fetus. Animal Reprod. Sci. 10:163-175, 1986.
Walton, A. and Hammond, J.: The maternal effects on growth and conformation in Shire horse-Shetland pony crosses. Proceed. Roy. Soc. B 125:311-335, 1938.
Wamsley, H., Alleman, R., Porter, M. and Long, M.: Cerebrospinal fluid findings in Florida horses with confirmed West Nile virus infection: 30 cases (2002). Vet. Pathol. 39:613 (abstract 2), 2002.
Whitwell, K.E.: Morphology and pathology of the equine umbilical cord. J. Reprod. Fertil. Suppl. 23:599-603, 1975.
Whitwell,
K.E. and Jeffcott, L.B.: Morphological studies on the fetal membranes
of the normal singleton foal at term. Res. Vet. Sci. 19:44-55, 1975.
Wieczorek, M., Switonski, M. and Yang, F.: A low-level X chromosome mosaicism
in mares, detected by chromosome painting. J. Appl. Genet. 42:205-209,
2001.
Williams, D.M., Smith, B.J., Donahue, J.M. and Poonacha, K.B.: Serological and microbiological findings on 3 farms with equine leptospiral abortions. Equine Vet. J. 26:105-108, 1994.
Wilsher, S. and Allen, W.R.: The effects of maternal age and parity on placental and fetal development in the mare. Equine Vet. J.: 35:476-483, 2003.
Wooding, F.B.P. and Flint, A.P.F.: Placentation. Chapter 4 (pp.233-460) in, G.E. Lamming, ed. Marshall's Physiology of Reproduction, 4th ed. Vol. 3, Part 1. Chapman & Hall, London, 1994.
Wooding, F.B.P. and Morgan, G.: Separate sites for placental transport of calcium and glucose in the equine placenta. J. Physiol. 507:47-48, 1988.
Wooding, F.B., Morgan, G., Fowden, A.L. and Allen, W.R.: Separate sites and mechanisms for placental transport of calcium, iron and glucose in the equine placenta. Placenta 21:635-645, 2000.
Wooding,
F.B.P., Morgan, G., Fowden, A.L. and Allen, W.R.: A structural and immunological
study of chorionic gonadotrophin production by equine trophoblast girdle
and cup cells. Placenta 22:749-767, 2001.
Woods, G.L., White, K.L., Vanderwall, D.K., Li, G.-P., Aston, K.I., Bunch,
T.D., Meerdo, L.N. and Pate, B.J.: A mule cloned from fetal cells by nuclear
transfer. Sciencexpress:www.sciencexpress.org/29 May 2003/page1/10.1126/science.1086743.
Zavy, M.T., Sharp, D.C., Bazer, F.W., Fazleabas, A., Sessions, F. and Roberts, R.M.: Identification of stage-specific and hormonally induced polypeptides in the uterine protein secretions of the mare during the oestrous cycle and pregnancy. J. Reprod. Fertil. 64:199-207, 1982.