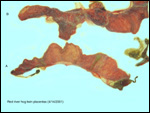
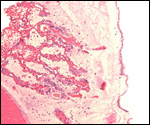
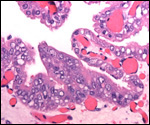
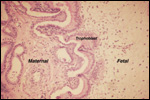
| |
9)
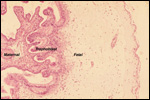
Trophoblast external to barrier
There is no uterine invasion by trophoblast at all.
10)
Endometrium
There is no decidualization during pregnancy in this bicornuate uterus
but endometrial ridge develop.
11)
Various features
None relevant to this species.
12)
Endocrinology
The reproductive cycle is similar to that of Sus scrofa (Eckstein
& Zuckerman, 1956). Cycles are about every 21 days, ovulation is spontaneous
(not induced), and estrus lasts 2-3 days. These authors also refer to
the estrogens and progestins in pregnancy. Progestins are low initially
but rise sharply with a peak at 11-15 days and then drop to near zero.
Estrogens rise towards term. The duration of pregnancy in the domestic
pig is between 112 and 115 days, longer in wild ancestors. Additional
information is available from Geisert (1998).
Berger et al. (2002) used fecal steroid metabolites to study the reproductive
physiologyy of warthogs, red river hogs and the babirusa. They were able
to define the length of cycles (35-37 days), identify non-cycling sows,
and monitor pregnancy.
13)
Genetics
The karyology of suids is still incomplete. The domestic pig has 38 chromosomes,
but Sus scrofa has two morphotypes (2n=36 or 38), and lower numbers
are found in giant forest hog (2n=32), warthog (2n=34). The "bushpig"
(red river hog) has 34 chromosomes (Melander & Hansen-Melander, 1980).
The sex chromosomes, as described by these authors, are unusual in their
morphology and differ from those of other suids. Specifically, the X-chromosomes
of the female specimen that these investigators studied differed in size
and appearance. Numerous karyotypes of different animals of red river
hogs in our laboratory have all shown 2n=34 and the X-chromosomes have
been large metacentric elements without any structural differences. Bosma
et al. (1991) had come to the same conclusion earlier. They studied a
"bushpig from the Duisburg Zoo (Germany) but did not indicate whether
it was a "red river hog" or another subspecies. Upon inquiry,
however, it was learned that the animal they studied was in fact a red
river hog. They found 34 chromosomes as well. Hybrids have not been described.
Marczynska & Pigon (1973) described an "African native pig"
karyotype (2n=38) but did not exactly specify the species. It is doubtful
that they had a bushpig for study.
Watanabe et al. (1985) have studied the mtDNA of European and Asiatic
pigs.
14)
Immunology
No relevant studies are known to us.
15)
Pathological features
There are a large number of diseases affecting swine, for instance hog
cholera and the African swine disease, a virus infection thought to have
originated from the carrier warthog. These and other aspects of pathological
states can be reviewed in Smith et al. (1972). Embryonic loss is common
in pigs, perhaps due to crowding. Geisert (1998) suggested that it may
be as frequent as 10-20% of embryos that are lost. Conjoined twin fetuses
have been observed, testifying to the occurrence of occasional monozygotic
twinning in domestic pigs.
16)
Physiological data
No physiologic studies have been carried out in this species; nevertheless,
numerous veterinary studies on domestic pigs have been done and can be
read in the relevant textbooks and in Geisert (1998). Fetal development
and pig anatomy are detailed by Patten (1947).
17)
Other resources
Cell strains are available through CRES
of the San Diego Zoo www.sandiegozoo.org
(CRES).
18)
Other features of interest
Domestic pigs have first been cloned in 2000. It would be of interest
whether the "resorbed" fetuses have chromosomal errors.
References
Berger, E.M., Leus, K. and Schwarzenberger, F.: Faecal steroid metabolites
for non-invasive assessment of reproduction in common warthogs (Phacochoerus
africanus), red river hogs (Potamochoerus porcus) and babirusa
(Babyrousa babyrussa celebensis). Pp. 411-412, In, Proceed. Europ.
Assoc. Zoo- and Wildlife Veterinarians, Heidelberg, May 8-12, 2002.
Björkman, N.: Fine structure of the fetal-maternal area of exchange
in the epitheliochorial and endotheliochorial types of placentation. Acta
anat. 86:1-22, 1973.
Bosma,
AA., deHaan, N.A. and MacDonald, A.A.: The current status of cytogenetics
of the Suidae: A review. Bongo 18:258-272, 1991.
Cell
strains of many specimens are available from CRES
at the San Diego Zoo: www.sandiegozoo.org.
Eckstein,
P. and Zuckerman, S.: The oestrus cycle in the mammalia. Chapter 4 (pp.
226-397), in Marshall's Physiology of Reproduction, 3rd ed. A.S. Parkes,
Ed. Longmans, Green and Co., London, 1956.
Geisert,
R.D.: Pigs. Pp. 792-799 in Vol. III of, Encyclopedia of Reproduction,
E. Knobil and J.D. Neill, eds., Academic Press, San Diego, 1998.
Grubb,
P.: The Afrotropical Suids Phaecochorus, Hylochchoerus,
and Potamochoerus. Chapter 4, pp. 66-75, In, Pigs, Peccaries and
Hippos. IUCN, Gland, Switzerland, 1993.
Heuser,
C.H.: A study of the implantation of the ovum of the pig from the stage
of the bilaminar blastocyst to the completion of the fetal membranes.
Contrib. Embyol. Carnegie Inst. 19:229-243, 1927.
Kingdon,
J.: East African Mammals. Vol. III, part B (Large Mammals). Academic Press,
N.Y. 1979.
Leister,
C.W.: The wild pigs of the world. Bull. N.Y. Zool. Soc. 42:121-130, 1939.
MacDonald,
A.A.: Uterine vasculature of the pregnant pig: A scanning electron microscope
study. Anat. Rec. 184:689-698, 1976.
MacDonald,
A.A. and Bosma, A.A.: Notes on placentation of Suina. Placenta 6:83-92,
1985).
Marczynska,
B. and Pigon, H.: Somatic chromosomes of the native African pig. Cytologia
38:111-116, 1973.
Melander,
Y. and Hansen-Melander, E.: Chromosome studies in African wild pigs (Suidae,
Mammalia). Hereditas 92:283-289, 1980.
Naaktgeboren,
C. and Zwillingberg, H.H.L.: Untersuchungen über die Auswüchse
am Amnion und an der Nabelschnur bei Walen, und Huftieren, mit besonderer
Berücksichtigung des europäischen Hausrindes. Acta Morphol.
Neerl.-Scand. 4:31-60- 1961.
Nowak,
R.M. and Paradiso, J.L.: Walker's Mammals of the World. 4th ed. Vol. II.
Johns Hopkins University Press, 1983.
Patten,
B.M.: The Embryology of the Pig. 2nd ed. The Blakiston Co. Philadelphia,
1931 (reprinted 1947).
Puschmann,
W.: Zootierhaltung.Vol. 2 Säugetiere. Deutscher Landwirtschaftsverlag,
Berlin, 1989.
Ramsey,
E. M.: The Placenta. Human and Animal. Praeger, N.Y., 1982.
Smith,
H.A., Jones, T.C. and Hunt, R.D.: Veterinary Pathology. Lea and Febiger,
Philadelphia, 1972.
Thenius,
E.: Zur Evolution und Verbreitungsgeschichte der Suidae (Artiodactyla,
Mammalia). Z. Säugetierk. 35:321-342, 1970.
Vercammen,
P., Seydack, A.H.W. and Oliver, W.L.R.: The bush pigs (Potamochoerus
porcus and P. larvatus). Pp. 93-101, In, Pigs, Peccaries and
Hippos. IUCN, Gland, Switzerland, 1993.
Watanabe,
T., Hayashi, Y., Ogasawara, N. and Tomoita, T.: Polymorphism of mitochondrial
DNA in pigs based on restriction endonuclease cleavage patterns. Biochem.
Genet. 23:105-113, 1985.
|