East
African River Hippopotamus
Hippopotamus amphibius kiboko
Pygmy Hippopotamus
Choeropsis (Hexaprotodon) liberiensis
Order: Artiodactyla
Family: Hippopotamidae
1) General Zoological Data
Hippopotamuses belong to the suborder Suina, a varied group of artiodactyls
that were summarized aptly by Macdonald and Bosma (1985) thus: "Apart
from a number of extinct groups, the artiodactyl suborder Suina comprises
the families Suidae (pigs), Tayassuidae (peccaries) and Hippopotamidae (hippopotami)
(Romer, 1966). The species which make up these families have several tens
of millions of years of separate evolutionary history and demonstrate wide
variation in body size, external and internal structure, behaviour, environmental
adaptation, and geographical distribution. Nevertheless, they are brought
together in one suborder, mainly because they share a primitive structure
of the limbs and dentition. In addition, they have no horns or antlers.
With regard to the soft parts, they have a relatively simple, non-ruminating
stomach (Langer, 1973)." One can add to this that they also share
very similar placentas.
The term hippopotamus derives from hippos=horse and potamos=river (Gotch,
1979), while amphibius makes reference to living on land and water. Choeropsis
refers to "belonging to a pig". "Kiboko" is the Swahili
name for the animal. "Hexaprotodon" refers to having 6 front teeth.
The plural of hippopotamus is hippopotamuses or hippopotami; the former
is employed here.
Numerous skeletal remains of extinct species indicate that hippos were widely
distributed over Africa and were also present in Cyprus, Madagascar and
reached into Palestine. Since hippos depend on river and water systems,
the desertification of Northern Africa has widely eliminated these species,
and human intervention has led to the elimination from Palestine, and Cyprus.
These finds have suggested to some authors that doubt exists for the need
to specify two genera for Hippopotamidae, an aspect that finds further support
from their essentially identical karyotypes. A comprehensive review of hippos
may be found in Eltringham (1999) and, for the Pygmy Hippopotamus, by Lang
(1975). Various authors have presented the action plans to assure survival
of these species into the future and the volume also gives the current distribution
of both species in Africa (Oliver, ed., 1993).
A number of genetic studies have recently been performed in attempts to
delineate the relationships of various artiodactyl taxa to cetacean and
other mammalian forms. Thus, a comprehensive analysis by Nikaido et al.
(1999) affirms the close relationship from their study of SINE in certain
certiodactyls. Ursing & Arnason (1998), studying the mitochondrial genomes,
had found support for a hippopotamus-cetacean clade, with a 54 MY separation.
Hasegawa & Adachi (1996) were critical of this analogy, and Nomura &
Yasue (1999) further pursued the taxonomic relations. Kleineidam et al.
(1999) studied pancreatic ribonuclease genes and also supported the relationship
of hippopotamidae to odontocetes. In a new study of the mitochondrial genome
of various presumably related taxa, Arnason et al. (2000) again confirmed
the cetacean-hippo clade and further examined the split of odontocetes and
mysticetes. Madsen et al. (2002) most recently examined the genetics of
adrenergic receptor molecules and found nesting of whales as a sister group
of hippos.
Hippopotamus
amphibius:
The large hippopotamus now is confined to the large river systems of Africa.
The animal is sometimes referred to as "Nile or River Hippo"
and weighs between 1,000 and 4,500 kg (Nowak, 1999). Gestation lasts 227-240
days and results in a single birth of 25-55 kg. The maximal lifespan is
given by Jones (in Nowak, 1999) as being 61 years. The gregarious animals
are very aggressive when disturbed during foraging and child rearing;
frequent deaths have been described (Pickles, 1987; Eltringham, 1999).
They are maintained in many zoological parks. The usage of subspecies
is not usually practiced any longer in designating origin of members of
this species.
Pygmy
hippopotamus:
This more terrestrial Western African species (mainly Liberia) is now
rare and in need of protection (Robinson, 1971). The subspecies Hexaprotodon
liberiensis heslopi has presumably become extinct (Antonius, 2003).
It is a solitary animal that is also frequently found in Zoos. It is long-lived;
Jones (1982) found a maximal lifespan of 43 years and 10 months. Adults
weigh 150-270 kg and neonates are 3-6 kg. The length of gestation is 184-204
days according to Nowak (1999), 200 days according to Lang (1975). The
latter author compiled the most extensive observations on the captive
management of pygmy hippopotamuses while director at Basel Zoo and observed
one set of stillborn twins (male/female) and he knew of one other set
of twins. He stated that animals with birth weights under 5 kg need special
attention.
 |
Nile hippopotamuses at San Diego Zoo. |
 |
Pygmy hippopotamus at San Diego Zoo. |
2)
General Gestational Data
The Nile hippopotamus has singleton births after a gestation period of 227-240
days. One placenta weighed 4,010 g. The pygmy hippopotamus also has singleton
births although Lang (1975) observed nonviable twins twice. Gestation lasts
200 days and the placentas obtained by me weighed 600, 950 and 950 g.
3)
Implantation
Really early stages of placentation have not been described. The only
young specimen studied in situ is that by Amoroso et al. (1958)
of a gestation estimated to be 60 days old. They found bare spots of membranes
at the peripheral tips and over the endocervical os. Moreover, the antimesometrial
villous covers were less profuse. That immature fetus was contained in
the left uterine horn, on which side the corpus luteum also was found.
4)
General Characterization of the Placenta
Two placentas of a Nile hippopotamus and four placentas from Pygmy hippopotamuses
were available to me. Because of their similarity, both species will be
presented in this chapter rather than separating them.
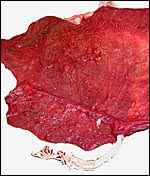
Nile
hippopotamus placenta:
The first placenta came from a gestation in which the father had killed
the neonate. The neonate weighed 32,500 g. The placenta was a huge sac,
covered diffusely with villi on its outside. It measured 104 cm in length
and 25 cm in width. It was 0.3 cm in thickness and had some non-villous
regions at each end. The umbilical cord inserted in the middle and measured
109 cm in length. It contained only three large blood vessels and an allantoic
duct. The placenta weighed 4,010 g. The fetal surface was covered with
a delicate amnion on which there were numerous plaques of squamous metaplasia
(pustules) that also extended over the entire length of the umbilical
cord.
The second placenta also comes from a term pregnancy in which the fetus
survived. It was accidentally born on land and, therefore, a good placenta
was available. It weighed 4,800 g, measured 130 cm in length and had a
four-vessel cord attached in the middle that was 31 cm long. From its
torn fetal end, however, extruded 51 cm torn vessels, much as they were
already depicted in the excellent description by Teuscher (1937).
Pygmy hippopotamus placenta:
The fetal membranes of a pygmy hippopotamus have previously been described by Teuscher (1937). I had four placentas available for study, three from term, surviving neonates and one from a neonatal death at San Diego Zoo. The male neonate that died weighed 5,430 g. Its placenta was 600 g; another placenta weighed 950 g, a fourth weighed 1,125 kg. It was 80 cm long and 0.3 cm in thickness. Its cord inserted centrally and was 100 cm long. The surface was uniformly studded with villi. The amnion had numerous areas of prominent squamous metaplasia.
5)
Details of fetal/maternal barrier
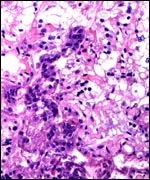
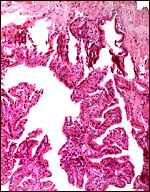
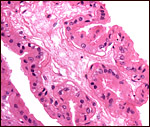
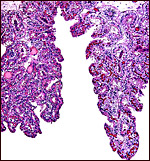
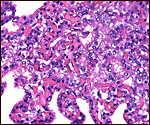
This is an epitheliochorial placenta with diffuse villous coverage over
nearly the entire chorion; only the lateral tips are free of villi. The
villi are short and only some of them are slightly branched. They are
covered by cuboidal trophoblast which contains no binucleated cells, as
seen in other artiodactyla. The subchorionic trophoblast between the villi
is rather more cylindrical and has a paler, empty-appearing cytoplasm.
Macdonald & Bosma (1985) described PAS-positive granules in their
cytoplasm. Syncytial cells are not present. Both species have yellow-brown
pigment accumulations in some trophoblastic cells, especially beneath
the chorionic plate. While this pigment is more frequently present between
the villi in the subchorionic trophoblast, it is worth noting that it
was also found in trophoblast of some of the villous tips. This is distinctly
different from the pigmented cells in the placentas of most other artiodactyla.
Moreover, it was not associated with any degenerative changes or areas
of hemorrhage. It is also iron-stain negative, as it is in other ungulates.
The second Nile hippo placenta had absolutely no yellow pigment in the
trophoblast. The villi contain no obvious Hofbauer cells, and the thick,
muscular nature of the chorionic vessels is remarkable.
Two types of villi are distinguished since their first descriptions by
Eschricht (1837) and von Baer (1837). They were further delineated by
Turner (1876), Ludwig (1968) and, again, by Macdonald & Bosma (1985).
They are the "areolar" villi that lie over the mouths of endometrial
glands, and the inter-areolar villi. The latter have more prominent interdigitations
of the fetal capillaries with the trophoblast than the former. The former
are less branched and broader, and Macdonald & Bosma described "plumes"
originating from some cells. Ludwig (1968) made a special study of these
villous differences in a variety of mammalian placentas and referred to
the former type as being "nephropneumoid", the other being the
"enteroid" villi. Turner (1876) had described in some detail
the surface structure of villi and endometrium, thus delineating the areolae
as regions of endometrial secretion that would subservice different exchange
functions than the more abundant nephropneumoid areas.
One remarkable feature is the intense interdigitation of the cuboidal
trophoblast with villous capillaries. This aspect has been specially considered
by Macdonald & Bosma (1985) who reviewed the literature and added
observations on two pygmy and one Nile hippopotamus placentas. Two of
these were of immature specimens. They make special reference to the different
appearance of some villi - those overlying the areolae (basal capillaries)
and those subserving a respiratory exchange (interdigitation of capillaries
with trophoblast). The latter produce the "epithelial plates",
regions of maximal thinness and, therefore, deemed to be used for gas
exchange.
6)
Umbilical cord
The umbilical cord of the hippopotamus has excited many earlier observers
because of their pronounced, large areas of squamous metaplasia, most beautifully
depicted by Hediger (1962). They are already present as foci of squamous
epithelium in very young specimens (Keibel, 1893). That author described
two young embryonic specimens, observed the areas of squamous metaplasia,
found the cord to be already quite long and lightly left spiraled, observed
the allantoic duct and remnants of the omphalomesenteric duct. He found
two arteries and two veins, aside from smaller vessels. The veins joined
immediately after entering the abdomen to form the single umbilical vein.
Amoroso et al. (1958), in their comprehensive description of placenta and
cord of an immature Nile hippopotamus placenta also observed 4 blood vessels.
It is thus surprising, that the umbilical cords of one of the one specimens
of Nile hippopotamus available to me and two of the pygmy hippopotamuses
all had only three blood vessels, in addition to the small number of small
blood vessels as are found in other artiodactyla. They were complete cross
sections of the midportions of the cords. This contrasts with the earlier
statements above and those of Macdonald and Bosma (1985) who also described
four vessels in both species of hippopotamus; they contrasted this to the
three vessels found in pigs and peccaries. One of the three pygmy hippopotamuses
available to me had four blood vessels. It is the neonate with funisitis
(see below). The other striking difference of hippos from suids, of course,
is their large number of "pustules" (squamous metaplasia) on cord
and amnion. Macdonald & Bosma (1985) referred to them as "pustule-encrusted"
cords. The pictures of these cords shown by Hediger (1962) are impressive.
It is probable that one blood vessel had already branched into the allantoic
sac in those cases where only three vessels were found. This is not too
surprising as the large vessels enter the allantoic sac and split off the
Wharton's jelly quite early. Thus, the isolated long vessels sticking out
from the end of the cord shown by Teuscher (1937) and in the Nile hippo
placenta shown above apparently lie more or less free until they find the
allantoic sac surface. The large areas of squamous metaplasia on the cord
that extend also for a short distance over the amnion are surprising because
they often contain clear fluid. Teuscher (1937) even found some bone in
one such cyst.
All observers also described the presence of numerous small blood vessels,
and Amoroso et al. (1958) quoted Wislocki as having suggested that these
contribute to the composition of amnionic fluid. It is true, however, that
there is no resolution as to nature and raison d'être for their existence.
While some accompany the allantoic duct, this is only the minority and it
will be seen in the section of the Nile hippo cord below that there are
relatively few blood vessels only in the cord, and that is equally true
of the allantoic sac.
The umbilical cord of hippos is quite long; thus, one of the pygmy hippopotamuses
had a 100 cm long cord and, grossly as well as histologically, it had only
three blood vessels. Another complete cord I observed also had only three
vessels and the allantoic duct with hippomanes, while a third had the normal
complement of four large vessels.
7)
Uteroplacental circulation
This has not been studied except for the cursory description of the one
pregnant uterus studied by Amoroso et al. (1958).
8)
Extraplacental membranes
The amnions of both species are thin and characterized by a very flat
epithelium that has a large number of "pearls" consisting of
squamous metaplasia with much keratin production. These extend also over
the entire umbilical cord. Brown hippomanes with birefringent crystalline
content are found in both species, in the allantoic duct as well as in
the allantoic sac. Keibel (1893) stated that the crystals were composed
of ammonium-magnesium phosphate. The hippomanes were the special topic
of a contribution by Hediger (1962) from his experience at Basel Zoo.
This is an interesting paper, but it confuses the squamous metaplasia
of cord and amnion with hippomanes, allantoic debris that is a concentrate
of urinary substances. I found no hippomanes in the second specimen of
Nile hippo.
There is a large allantoic sac that partially separates amnion from chorion.
It has cuboidal epithelium that was mostly eroded in my specimen. Remarkably,
there are very sparse vessels in the allantoic membrane. Amoroso et al.
(1958) described it as being bilobed and entered centrally by the allantoic
duct from the umbilical cord. It contained hippomanes even in the young
specimens observed by earlier observers. The lining is a flat squamoid
epithelium. Near term, the allantoic sac is smaller and gives way to a
larger amnion.
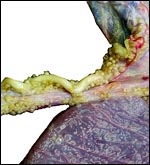
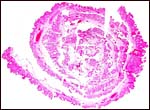
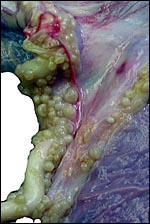
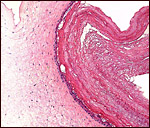
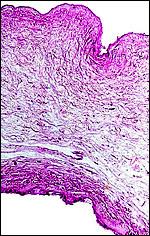 |
Allantoamnion of Nile River Hippopotamus. Amnion above, allantoic sac below. |
9)
Trophoblast external to barrier
In the absence of an implanted placenta for study, it can only be conjectured
that, analogous to other artiodactyla and cetacea, trophoblastic invasion
into endometrium does not occur.
10)
Endometrium
Again, in the absence of an implanted placenta, the endometrium and possible
decidualization cannot be described. Obviously, there is no decidua capsularis
as the entire sac is covered by villi. The endometrium underlying the
villi was intact and covered by cuboidal epithelium in the specimen described
by Amoroso et al. (1958). They also reported on a significant infiltration
by lymphocytes and plasma cells of the endometrium.
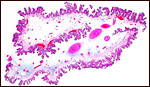
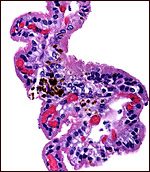
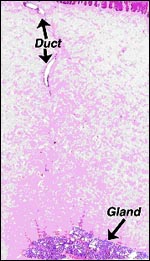
The neonatal endometrium is not stimulated and has prominent ridges, but
no endometrial glands.
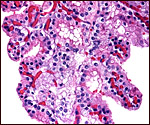
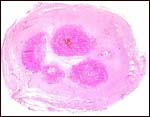
| Endometrium of neonatal pygmy hippopotamus. |
11)
Various features
Both species have a peculiar skin secretion that has often been referred
to as "blood sweat" because it appears sometimes similar to blood
when the light shines appropriately (Nowak , 1999). It is a clear, mucoid
secretion that makes the skin slippery and only appears red on occasion.
We have attempted to identify specific pigments (the fluid turns dark after
standing), without success. It is conjectured that this "sweat"
is produced to allow the skin to remain moist, thus avoiding cracking of
the epidermis when the animal is out of the water. In sections taken from
injuries, rare, deeply-seated glands were found in a Nile river hippopotamus
of one year age. It also contained a thin duct. In a 39 y.o. pygmy hippopotamus
one hair shaft were found. The deeply-seated glands have minute PAS-positive
granules in occasional cells and more in the ducts, whose content is also
PAS-positive. They are mucin-negative and did not stain with BRST and SMA
(smooth muscle antigen) antibodies either. There were additional sebaceous
glands surrounding the hair shafts of the pygmy hippopotamus. None of the
contents were discolored as "blood sweat" is. Photographs of these
findings are shown next. Oliver (1975) has written about these gland and
referred to earlier literature. They were shown to produce hyperosmolar,
alkaline secretion whose role "remains obscure". He observed that
the physiology and structure of these glands is essentially similar in both
species. Lochte (1951) described in great detail the structure of hippopotamus
hair. The tusks of both species grow continuously and have been used as
"ivory". Nursing of neonates takes place under water. Ears are
moved independently, and defecation is dispersed by intense movement of
the tail.
The nature of the pigment in “blood sweat” has now been elucidated by Saikawa et al. (2004) in a detailed chemical analysis. They identified an orange and red pigment. The labile red pigment being hipposiduric acid (so named by the authors), the orange pigment is norhipposudric acid, three-phenolic rings structures. The red pigment has some antibiotic property and the possibility of the pigments acting as sunscreens was proposed by the authors. They are quite labile and are under further study by the authors.
12) Endocrinology
In 1975, Lang stated that he knew of no means by which pregnancy could be diagnosed in the pygmy hippopotamus other than by the absence of copulation. Graham et al. (2002a) were the first investigators to study the endocrine aspects of ovulation and pregnancy in Nile hippopotamus from fecal excretions of progesterone metabolites. They determined the cycle length to be 35.3 days, and higher progestagen levels were found during pregnancy than after ovulation. Usually, lactation prevented ovulation. Cycles during the 3rd and 4th years of life indicated that pregnancies ensued after the fourth cycle. In another study (Graham et al., 2002b) the authors examined the effect of Depo-Provera.
The ovaries of the Nile hippopotamus were described as partially enclosed in a membranous bursa (Laws & Clough, 1966). They also found accessory corpora lutea in pregnancy that regressed during lactation.
13)
Genetics
The Nile hippopotamus and the pygmy hippopotamus, both have 36 chromosomes,
all metacentrics (Gerneke, 1965; Hsu & Benirschke, 1977). Only the
Y-chromosome is acrocentric and it is larger in the pygmy hippopotamus.
The two karyotypes are otherwise essentially similar. Hybrids are unknown.
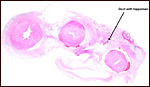
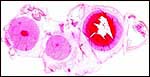
 |
Karyotype of male Nile hippopotamus. |
 |
Karyotype of female Nile hippopotamus. |
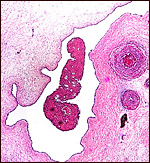
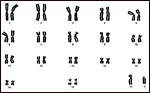 |
Karyotype of male pygmy hippopotamus. |
14)
Immunology
I am not aware that any studies have been done of the hippopotamus immune
system other than those described in the paper by Tsuji et al. (1984). These
authors studied the molecular weights of transferrins by rabbit antibodies
in a variety of artiodactyl mammals.
15)
Pathological features
Bush et al. (1972) described uterine prolapse in a pygmy hippopotamus.
Schistosoma edwardiensis was found by Pitchford & Visser (1981)
at Kruger National Park in the large hippopotamus, and they discussed
the synonymy of different schistosoma species. Dauth et al. (1988) studied
blood levels in river hippopotamuses of the Kruger National Park and found
them to be lower to that of the population. Copper concentrations (and
other trace elements) in liver and kidney were studied by Mwase et al.
(2002) at Kafue National Park. No elevation was found, irrespective of
the proximity to the "copperbelt". Fatal encephalomyocarditis
virus infection of a pygmy hippopotamus (and other species) was described
by Reddacliff et al. (1997) from Australia. Kuttin et al. (1982) described
"coccidiosis of the placenta" in a Nile hippopotamus, but the
paper was not available to me. Griner (1983) examined only neonates of
Nile hippopotamuses that had drowned because of management errors. Two
neonatal pygmy hippopotamuses (one stillborn) had unknown causes of death.
A variety of parasites and diseases of hippopotamuses is discussed by
Eltringham (1999
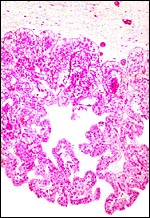
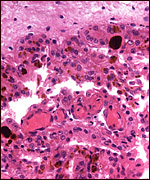
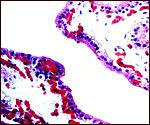
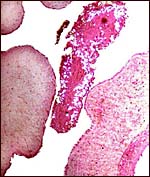
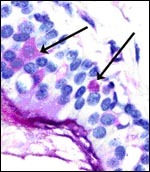
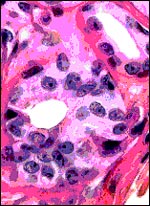
One of our pygmy hippopotamus specimens had marked funisitis (inflammation
of arteries and veins) of the umbilical cord, in the absence of chorioamnionitis,
as would be usually seen in human specimens. Organisms were not seen histologically,
but the neonate had intrauterine aspiration pneumonia. A similar situation
has been observed in ascending infection of the horse.
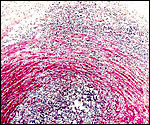 |
Umbilical phlebitis of a pygmy hippopotamus umbilical cord. |
16)
Physiologic data
Thermoregulation of the hippopotamus was studied by Cena (1964). Macdonald
& Hartman (1983) dissected the stomachs of adult and neonatal pygmy
hippos, finding them to be four-chambered. Griner (1983) specified only
three chambers. Nevertheless, the animals do not ruminate. The renal anatomy
of adult and neonatal pygmy hippopotamus was described in great detail by
Maluf (1994). Rouille et al. (1988) studied the occurrence of vasopressin
in river hippopotamus and in collared peccary. They found arginine vasopressin
but no lysine vasopressine in these species, contrary to the finding in
Suidae. In a molecular study of milk casein, Gatesy et al. (1996) found
the structure to be closer to cetacea than to other artiodactyl species,
confirming the close relationship of whales and hippos. From these data,
the authors suggested that an intermediate fossil may so far be "missing".
The means of immobilization of the Nile hippopotamus (9 animals) was described
by Ramsay et al. (1998). One death occurred from apnea. Flach et al. (1998)
removed a dead fetus from a pygmy hippo by Cesarean section with recovery
of the dam. Using specific antibodies to retinal cone visual pigments, Peichl
et al. (2001) demonstrated the absence of S-cones in odontocetes and five
species of seals, but affirmed their presence in pygmy hippopotamus (and
other terrestrial animals). This indicated to the authors an absence of
color vision in marine mammals. Eltringham (1999) has most comprehensively
detailed the anatomy, known physiology, distribution, and general problems
affecting hippopotamuses.
17)
Other resources
Cell strains of fibroblast are available of both species from CRES
at the San Diego Zoo by contacting Dr. Oliver Ryder at oryder@ucsd.edu.
18) Other remarks - What additional Information is needed?
The discrepancy of the number of umbilical cord blood vessels is troubling
and needs further study. Also, early specimens, preferably implanted placentas,
should be described. There is too little endocrine information and the
absence of binucleated trophoblastic cells is of interest. Thus, is there
any placental lactogen produced during pregnancy, as in other ungulates?
Ascending infection, as seen in equines, needs to be paid attention to.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego.
References
Amoroso, E.C., Hancock, N.A. and Kellas, L.: The foetal membranes and
placenta of the hippopotamus (Hippopotamus amphibius (Linnaeus).
Proc. Zool. Soc. London 130:437-454, 1958.
Antonius, E.: Lexikon ausgerotteter Vögel und Säugetiere. Natur
und Tier - Verlag, Munster, 2003.
Arnason, U., Gullberg, A., Gretarsdottir, S., Ursing, B. and Janke, A.: The mitochondrial genome of the sperm whale and a new molecular reference for estimating eutherian divergence dates. J. Mol. Evol. 50:569-578, 2000.
Baer, K.E.v.: Ueber Entwicklungsgeschichte der Thiere. 1837.
Bush, M., Lemken, R. and Moore, J.A.: Prolapsed uterus in a pigmy hippopotamus. J. Amer. Vet. Med. Ass. 161:651, 1972.
Cena, K.: Thermoregulation in the hippopotamus. Int. J. Biometeorol. 8:57-60, 1964.
Dauth, J., Dreyer, M.J., Raubenheimer, E.J., Lemmer, L.B. and de Vos, V.: Blood lead concentrations in hippopotami (Hippopotamus amphibius) in the Kruger National Park. J.S. Afr. Vet. Assoc. 59:153-154, 1988.
Eltringham, S.K.: The Hippos. Academic Press, London. 1999.
Eschricht, D.F.: De organis, quae respirationi et nutritioni foetus mammalium inserviunt: anniversaria (Schultziana, Havniae). 1837 {quoted from Ludwig, 1968].
Flach, E.J., Furrokh, I.K., Thornton, S.M., Smith, J., Parkyn, J.P. and Campbell, E.J.: Caesarean section in a pygmy hippopotamus (Choeropsis liberiensis) and the management of the wound. Vet. Rec. 143:611-613, 1998.
Gatesy, J., Hayashi, C., Cronin, M.A. and Arctander, P.: Evidence from milk casein genes that cetaceans are close relatives of hippoptamid artiodactyls. Mol. Biol. Evol. 13:954-963, 1996.
Gerneke, W.H.: The chromosomes and neutrophils nuclear appendages of Hippopotamus amphibius Linnaeus 1758. Onderstepoort J. Vet. Res. 32:181-186, 1965.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Graham, L.H., Reid, K., Webster, T., Richards, M. and Joseph, S.: Endocrine patterns associated with reproduction in the Nile hippopotamus (Hippopotamus amphibius) as assessed by fecal progestagen analysis. Gen. Comp. Endocrinol. 128:74-81, 2002a.
Graham, L.H., Webster, T., Richards, M., Reid, K. and Joseph, S.: Ovarian function in the Nile hippopotamus and the effects of Depo-Provera administration. Reprod. Suppl. 60:65-70, 2002b.
Griner, L.A.: Pathology of Zoo Animals. Zoological Society of San Diego, San Diego, California, 1983.
Hasegawa, M. and Adachi, J.: Phylogenetic position of cetaceans relative to artiodactyls: reanalysis of mitochondrial and nuclear sequences. Mol. Biol. Evol. 13:710-717, 1996.
Hediger, H.: Die Hippomanes der Hippopotamiden. Zool. Garten 26:321-336, 1962.
Hsu, T.C. and Benirschke, K.: An Atlas of Mammalian Chromosomes. Volume 10, Folio 507, 1977. Springer-Verlag, N.Y.
Jones, M.L.: Longevity of captive mammals. Zool. Garten 52:113-128, 1982.
Keibel, F.: Ueber den Nabelstrang des Nilpferdes. Anat. Anzeiger 8:497-504, 1893.
Kleineidam, R.G., Pesole, G., Breukelman, H.J., Beintema, J.J. and Kastelein, R.A.: Inclusion of cetaceans within the order Artiodactyla based on phylogenetic analysis of pancreatic ribonuclease genes. J. Mol. Evol. 48:360-368, 1999.
Kuttin, E.S., Loupal, G., Kohler, H. and Supperer, R.: Coccidiosis of the placenta in a hippopotamus (Hippopotamus amphibius). 29:153-159, 1982.
Lang, E.M.: Das Zwergflusspferd Choeropsis liberiensis. A Ziemsen Verlag, Wittenberg Lutherstadt, 1975.
Laws,
R.M. and Clough, G.: Observations on reproduction in the hippopotamus,
Hippopotamus amphibius Linn. Pp. 117-140, in, I.W. Rowlands, ed.:
Comparative Biology of Reproduction in Mammals. Sympos. Zool. Soc. London
# 15. Academic Press, London, 1966.
Lochte, Th.: Untersuchungen an Haaren eines neugeborenen Nilpferdes und
eines Flusspferdes. Zool. Garten 18:119-124, 1951.
Ludwig, K.S.: Zur vergleichenden Histologie des Allantochorion. Revue Suisse Zool. 75:819-831, 1968.
Macdonald, A.A. and Bosma, A.A.: Notes on placentation in the Suina. Placenta 6:83-91, 1951.
Macdonald, A.A. and Hartman, W.: Comparative and functional morphology of the stomach in the adult and newborn pigmy hippopotamus (Choeropsis liberiensis). J. Morphol. 177:269-276, 1983.
Madsen, O., Willemsen, D., Ursing, B.M., Arnason, U. and de Jong, W.W.: Molecular evolution of the mammalian alpha 2b adrenergic receptor. Mol. Biol. Evol. 19:2150-2160, 2002.
Maluf, N.S.: Renal anatomy of the pigmy hippopotamus (Choeropsis liberiensis): an overview. Anat. Histol. Embryol. 23:189-206, 1994.
Mwase, M., Almli, B., Sivertsen, T., Musonda, M.M. and Flaoyen, A.: Hepatic and renal concentrations of copper and other trace elements in hippopotami (Hippopotamus amphibius L) living in and adjacent to the Kafue and Luangwa Rivers in Zambia. Onderstepoort J. Vet. Res. 69:207-214, 2002.
Nikaido, M., Rooney, A.P. and Okada, N.: Phylogenetic relationships among certiodactyls based on insertion of short and long interspersed elements: Hippopotamuses are closest extant relatives of whales. Proc. Natl. Acad. Sci. USA 96:10261-10266, 1999.
Nomura, O. and Yasue, H.: Genetic relationships among hippopotamus, whales, and bovine based on SINE insertion analysis. Mamm. Genome 10:526-527, 1999.
Nowak,
R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press,
Baltimore, 1999.
Oliver, R.C.D.: Aspects of skin physiology in the Pigmy hippopotamus Choeropsis
liberiensis. J. Zool. London 176:211-213, 1975.
Oliver,
W.L.R., ed. Pigs, Peccaries, and Hippos. IUCN/SSC Pigs & Peccaries,
and Hippo Specialist Groups. IUCN Gland, Switzerland, 1993.
Peichl, L., Behrmann, G. and Kroger, R.H.: For whales and seals the ocean
is not blue: a visual pigment loss in marine mammals. Eur. J. Neurosci.
13:1520-1528, 2001.
Pickles, G.: Injuries by wild animals in the African bush. J.R. Army Med. Corps 133:159-160, 1987.
Pitchford, R.J. and Visser, P.S.: Schistosoma Weinland, 1958 from Hippopotamus amphibius Linnaeus, 1758 in the Kruger National Park. Onderstepoort J. Vet. Res. 48:181-184, 1981.
Ramsay, E.C., Loomis, M.R., Mehren, K.G., Boardman, W.S., Jensen, J. and Geiser, D.: Chemical restraint of the Nile hippopotamus (Hippopotamus amphibius) in captivity. J. Zoo Wildl. Med. 29:45-49, 1998.
Reddacliff, L.A., Kirkland, P.D., Hartley, W.J. and Reece, R.L.: Encephalomyocarditis virus infections in an Australian zoo. J. Zoo Wildl. Med. 28:153-157, 1997.
Robinson, P.T.: Wildlife trends in Liberia and Sierra Leone. Oryx 11:117-122, 1971.
Rouille,
Y., Chauvet, M.T., Cauvet, J., Acher, R. and Hadley, M.E.: The distribution
of lysine vasopressin (lysipressine) in placental mammals: a reinvestigation
of the Hippopotamidae (Hippopotamus amphibius) and Tayassuidae
(Tayassu angulatus) families. Gen. Comp. Endocrinol. 71:475-483,
1988.
Saikawa, Y., Hashimoto, K., Nakata, M., Yoshihara, M., Nagai, K., Ida, M. and Komiya, T.: The red sweat of the hippopotamus. The red and orange pigments in the secretion account for its protective properties. Nature 429:363, 2004.
Teuscher, R.: Anatomische Untersuchungen über die Fruchthüllen
des Zwergflusspferdes. Z. Anat. 555, 1937.
Tsuji, S., Kato, H., Matsuoka, Y., Fukushima, T., Nanjoh, I., Amano, T. and Namikawa, T.: Phylogenetical and ontogenetical studies on the molecular weight heterogeneity of bovine serum transferrin. Biochem. Genet. 22:1127-1143, 1984.
Turner, W.: On the structure of the diffused, the polycotyledonary and the zonary forms of placenta. J. Anat. Physiol. 10:127-177, 1876.
Ursing, B.M. and Arnason, U.: Analyses of mitochondrial genomes strongly support a hippopotamus-whale clade. Proc. Royal Soc. London B Biol. Sci. 265:2251-2255, 1998.