|
(Clicking
on the thumbnail images below will launch a new window and a larger
version of the thumbnail.)
|
Hemitragus jemlahicus
Order: Artiodactyla
Family: Bovidae
1) General Zoological Data
There are three recognized species of tahrs: Hemitragus jemlahicus, the Himalayan tahr which is also the best-known species and discussed here, then H. jayakuri, the Arabian or Oman tahr, and finally H. hylocrius, the Nilgiri tahr from southern India (Nowak, 1999). One specimen of that species has been studied by me and can be viewed in the separate chapter on Nilgiri Tahr. The Himalayan tahr whose pregnant uterus I was able to study resided at the San Diego Zoo, where a successful colony is kept. The term "tahr" is reported to be Nepalese for this species (Gotch, 1979).
Tahrs may have derived from the ancestral form Myotragus balearicus, according to Lalueza-Fox et al. (2000) who studied the mtDNA of that species. Tahrs are said to weight between 39 and 100 kg. The Himalayan tahr has been introduced to New Zealand where it is doing extremely well. The longevity of the Himalayan tahr is over 21 years according to Jones (1993). Regrettably the pictures available to me of this species do not do it justice, as a glance at Nowak (1999) for instance will disclose. The typical adult Himalayan tahr has a much more pronounced, beautiful mane.
 |
Pair of Himalayan Tahr at San Diego's Wild Animal Park. |
 |
Another Himalayan Tahr at the Wild Animal Park. |
Himalayan tahrs generally have single births, but twins occur occasionally. Their gestation lasts 6 months (Brown, 1936), and Nowak (1999) stated a period of 177 to 242 days.
3)
Implantation
Early stages of implantation have not been described.
4)
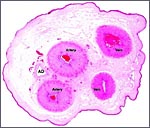
General Characterization of the Placenta
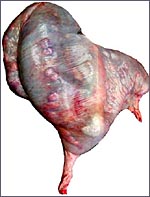
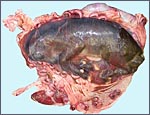
One specimen from a pregnant tahr was available. The dam had to be euthanized
because of a broken foot. She was in the last month of gestation with
a female fetus weighing 2,400 g that occupied the right uterine horn.
The placenta, however, extended into both horns and had 70 cotyledons
measuring between 1.2 and 3.5 cm in diameters. They had depressed center
and are thus "concave" according to Mossman's definition (1987).
Uterus and implanted placenta together weighed 1,100 g. The number of
cotyledons is thus considerably greater than that of the Nilgiri Tahr.
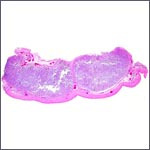
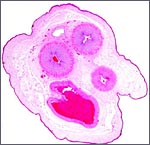
This is a cotyledonary epithelio-chorial placenta with long, sparsely-branched villi interdigitating with the endometrium. The trophoblast is cuboidal with the exception of the prominent and numerous binucleate cells. The presumed secretory product of these binucleate cells has been discussed in several other chapters on ungulate placentas (Cretan Goat). It is presumed to be placental lactogen. Only in a few areas, especially central in the cotyledons, is there focal superficial infiltration of trophoblast into the endometrium, establishing focally a syndesmochorial relationship. Throughout this placenta were areas of calcification in the endometrial spaces that were not found in the Nilgiri tahr placenta but were found in the ibex placentas. The subchorionic trophoblast has large quantities of yellow pigment that is iron-stain negative, as was the case in Nilgiri Tahr. As has also been discussed in other chapters, it may represent storage of melanin (for a more detailed discussion see the chapter on Alpine Ibex).
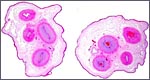
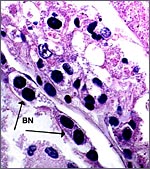 |
Binucleate trophoblast on villous surface. |
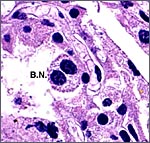 |
Another binucleate trophoblast. |
The umbilical cord measured 9 cm and had four blood vessels and an allantoic duct. Characteristically, the duct is centrally located between the two umbilical arteries. The cord's surface had numerous tiny granules of squamous metaplasia. It was not spiraled. Microscopically it is remarkable to witness that the smaller blood vessels come only from the larger umbilical arteries, not the veins, and that they are strikingly congregated around the allantoic duct.
No studies have been undertaken.
8)
Extraplacental membranes
The very thin amnionic sac had numerous tiny granules of squamous metaplasia.
It is typically avascular and lies within the large allantoic sac which
is covered with columnar epithelium. The allantois also has a rich vascular
network.
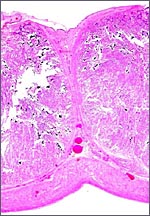
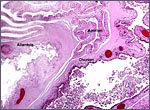 |
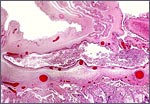
At the left edge of a cotyledon the various layers of the membranes insert. |
With the focal exception of a small area of trophoblastic infiltration of endometrium in the cotyledonary centers, there is no trophoblastic invasion and trophoblast does not reach the myometrium or blood vessels.
10)
Endometrium
The endometrium is flattened, has glands at the edges of, and between
the cotyledons. Typical decidualization does not seem to occur.
11) Various features
The Himalayan tahr, as so many other ungulates, also has a strong production
of very viscid mucus in the endocervix. There is massive glandular distention
in the endocervix and the mucus presumably plays the same role as in other
mammals. I believe that it is the most effective barrier to ascending
infection from the vaginal canal.
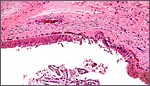
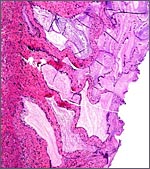 |
Endocervical canal with much mucus production by the endocervical glands. |
A single corpus luteum was present on the side of the pregnant horn. We have not undertaken endocrine studies and I am not aware of any publications on this topic.
13) Genetics
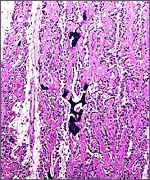
The Himalayan tahr has 48 chromosomes whose karyotype is shown below (Benirschke & Kumamoto, 1980; see also Nelson-Rees et al., 1967 and Bunch & Nadler, 1980). The Nilgiri tahr, on the other hand, has 58 chromosomes, as does the Arabian tahr. The study by Bunch & Nadler (1980) on Himalayan tahrs was undertaken with the Giemsa banding method and it compared the results with many other caprine species. They concluded from this investigation that chromosomal fusion, rather than fission, best explains the evolutionary relationships and chromosomal rearrangements of caprinae. This is also my interpretation. Hybrids among tahr species have not been reported to my knowledge, and hybrids of tahrs with goats have resulted in abortions, but in no live offspring (Gray, 1972).
 |
Himalayan Tahr karyotype with 2n=48. |
I am not aware of any publications other than those relating to the virologic studies listed next.
15)
Pathological features
There is only a sparse literature on Himalayan tahr pathology. Semevolos
et al. (1998) reported on one case of shoulder joint dislocation, among
a variety of other large hoofed stock. Two distinct caprine arthritis
encephalitis viruses have been isolated from Himalayan tahr cell lines
(Dahlberg et al., 1981; Kalinski et al., 1994; see also Archambault et
al., 1994) that were later genetically analyzed by the same investigators
(Gazit et al., 1996). Nielsen et al. (1988) described what they assumed
to be malignant catarrhal fever virus infection of Nilgiri tahr, but they
did not study Himalayan tahrs.
16)
Physiologic data
No such studies have been conducted.
17)
Other resources
Cell strains of fibroblasts from numerous animals are available from CRES
at the San Diego Zoo though contacting Dr. Oliver Ryder at oryder@ucsd.edu.
Other investigators cited here have used other cell lines for their virus
study.
18)
Other remarks - What additional Information is needed?
Endocrine studies are badly needed.
Acknowledgement
The animal photographs in this chapter come from the Zoological Society
of San Diego. I appreciate also very much the help of the pathologists
at the San Diego Zoo.
References
Archambault, D., Stein, C.A. and Cohen, J.S.: Phosphorothioate oligonucleotides
inhibit the replication of lentiviruses and type D retroviruses, but not
that of type C retroviruses. Arch. Virol. 139:97-109.
Benirschke, K. and Kumamoto, A.T. The chromosomes of the Nilgiri tahr Hemitragus hylocrius. Intern. Zoo. Yearbk. 20:274-275, 1980.
Brown, C.E.: Rearing wild animals in captivity and gestation periods. J. Mammal. 11:303-305, 1936.
Bunch, T.D. and Nadler, C.F.: Giemsa-band patterns of the tahr and chromosomal evolution of the tribe Caprini. J. Hered. 71:110-116, 1980.
Dahlberg, J.E., Gaskin, J.M. and Perk, K.: Morphological and immunological comparison of caprine arthritis encephalitis and ovine progressive pneumonia viruses. J. Virol. 39:914-919, 1981.
Gazit, A., Mashiah, P., Kalinski, H., Gast, A., Rosin-Abersfeld, R., Tronick, S.R. and Yaniv, A.: Two species of Rev proteins, with distinct N termini, are expressed by caprine arthritis encephalitis virus. J. Virol. 70:2674-2677, 1996.
Gotch, A.F.: Mammals - Their Latin Names Explained. Blandford Press, Poole, Dorset, 1979.
Gray, A.P.: Mammalian Hybrids. A Check-list with Bibliography. 2nd edition. Commonwealth Agricultural Bureaux Farnham Royal, Slough, England, 1972.
Jones, M.L.: Longevity of ungulates in captivity. Intern. Zoo Yearbk. 32:159-169, 1993.
Kalinski, H., Mashiah, P., Rotem, D., Orzech, Y., Sherman, L., Miki, T., Yaniv, A., Gazit, A. and Tronick, S.R.: Characterization of cDNAs species encoding the Tat protein of caprine arthritis encephalitis virus. Virology 204:828-834, 1994.
Lalueza-Fox, C., Bertranpetit, J., Alcover, J.A., Shailer, N. and Hagelberg, E.: Mitochondrial DNA from Myotragus balearicus, an extinct bovid from the Balearic Islands. J. Exp. Zool. 288:56-62, 2000.
Mossman, H.W.: Vertebrate Fetal Membranes. MacMillan, Houndmills, 1987.
Nelson-Rees, W.A., Kniazeff, A.J., Malley, R.L. and Darby, N.B. Jr.: On
the karyotype of the tahr Hemitragus jemlahicus and the Y-chromosome
of goats and sheep. Chromosoma 23:154-161, 1967.
Nielsen, N.O., Oosterhuis, J., Janssen, D., McColl, K., Anderson, M.P. and Heuschele, W.P.: Fatal respiratory disease in Nilgiri tahr: possibly malignant catarrhal fever. Can. J. Vet. Res. 52:216-221, 1988.
Nowak, R.M.: Walker's Mammals of the World. 6th ed. The Johns Hopkins Press, Baltimore, 1999.
Semevolos, S.A., Nixon, A.J., Goodrich, L.R. and Ducharme, N.G.: Shoulder joint luxation in large animals: 14 cases (1976-1997). J. Amer. Vet. Med. Assoc. 213:1608-1611, 1998.